Stathmin protein level, a potential predictive marker for taxane treatment response in endometrial cancer
- PMID: 24587245
- PMCID: PMC3934991
- DOI: 10.1371/journal.pone.0090141
Stathmin protein level, a potential predictive marker for taxane treatment response in endometrial cancer
Abstract
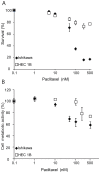
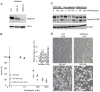
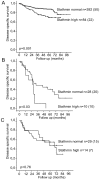
Stathmin is a prognostic marker in many cancers, including endometrial cancer. Preclinical studies, predominantly in breast cancer, have suggested that stathmin may additionally be a predictive marker for response to paclitaxel. We first evaluated the response to paclitaxel in endometrial cancer cell lines before and after stathmin knock-down. Subsequently we investigated the clinical response to paclitaxel containing chemotherapy in metastatic endometrial cancer in relation to stathmin protein level in tumors. Stathmin level was also determined in metastatic lesions, analyzing changes in biomarker status on disease progression. Knock-down of stathmin improved sensitivity to paclitaxel in endometrial carcinoma cell lines with both naturally higher and lower sensitivity to paclitaxel. In clinical samples, high stathmin level was demonstrated to be associated with poor response to paclitaxel containing chemotherapy and to reduced disease specific survival only in patients treated with such combination. Stathmin level increased significantly from primary to metastatic lesions. This study suggests, supported by both preclinical and clinical data, that stathmin could be a predictive biomarker for response to paclitaxel treatment in endometrial cancer. Re-assessment of stathmin level in metastatic lesions prior to treatment start may be relevant. Also, validation in a randomized clinical trial will be important.
Conflict of interest statement
Figures


References
-
- Belletti B, Baldassarre G (2011) Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert opinion on therapeutic targets 15: 1249–1266. - PubMed
-
- Rubin CI, Atweh GF (2004) The role of stathmin in the regulation of the cell cycle. Journal of cellular biochemistry 93: 242–250. - PubMed
-
- Biomarkers Definitions Working G (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clinical pharmacology and therapeutics 69: 89–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

