Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis
- PMID: 24587351
- PMCID: PMC3938718
- DOI: 10.1371/journal.pone.0090398
Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis
Abstract
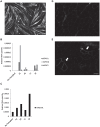
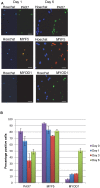
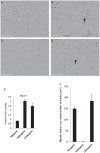
Satellite cells are the chief contributor to skeletal muscle growth and regeneration. The study of mouse satellite cells has accelerated in recent years due to technical advancements in the isolation of these cells. The study of human satellite cells has lagged and thus little is known about how the biology of mouse and human satellite cells compare. We developed a flow cytometry-based method to prospectively isolate human skeletal muscle progenitors from the satellite cell pool using positive and negative selection markers. Results show that this pool is enriched in PAX7 expressing cells that possess robust myogenic potential including the ability to give rise to de novo muscle in vivo. We compared mouse and human satellite cells in culture and identify differences in the elaboration of the myogenic genetic program and in the sensitivity of the cells to cytokine stimulation. These results indicate that not all mechanisms regulating mouse satellite cell activation are conserved in human satellite cells and that such differences may impact the clinical translation of therapeutics validated in mouse models. Thus, the findings of this study are relevant to developing therapies to combat muscle disease.
Conflict of interest statement
Figures



References
-
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, et al. (2004) Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119: 543–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

