Optimizing recellularization of whole decellularized heart extracellular matrix
- PMID: 24587354
- PMCID: PMC3937369
- DOI: 10.1371/journal.pone.0090406
Optimizing recellularization of whole decellularized heart extracellular matrix
Abstract
Rationale: Perfusion decellularization of cadaveric hearts removes cells and generates a cell-free extracellular matrix scaffold containing acellular vascular conduits, which are theoretically sufficient to perfuse and support tissue-engineered heart constructs. However, after transplantation, these acellular vascular conduits clot, even with anti-coagulation. Here, our objective was to create a less thrombogenic scaffold and improve recellularized-left ventricular contractility by re-lining vascular conduits of a decellularized rat heart with rat aortic endothelial cells (RAECs).
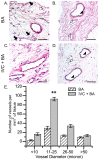
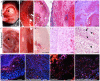
Methods and results: We used three strategies to recellularize perfusion-decellularized rat heart vasculature with RAECs: retrograde aortic infusion, brachiocephalic artery (BA) infusion, or a combination of inferior vena cava (IVC) plus BA infusion. The re-endothelialized scaffolds were maintained under vascular flow in vitro for 7 days, and then cell morphology, location, and viability were examined. Thrombogenicity of the scaffold was assessed in vitro and in vivo. Both BA and IVC+BA cell delivery resulted in a whole heart distribution of RAECs that proliferated, retained an endothelial phenotype, and expressed endothelial nitric oxide synthase and von Willebrand factor. Infusing RAECs via the combination IVC+BA method increased scaffold cellularity and the number of vessels that were lined with endothelial cells; re-endothelialization by using BA or IVC+BA cell delivery significantly reduced in vitro thrombogenicity. In vivo, both acellular and re-endothelialized scaffolds recruited non-immune host cells into the organ parenchyma and vasculature. Finally, re-endothelialization before recellularization of the left ventricular wall with neonatal cardiac cells enhanced construct contractility.
Conclusions: This is the first study to re-endothelialize whole decellularized hearts throughout both arterial and venous beds and cavities by using arterial and venous delivery. The combination (IVC+BA) delivery strategy results in enhanced scaffold vessel re-endothelialization compared to single-route strategies. Re-endothelialization reduced scaffold thrombogencity and improved contractility of left ventricular-recellularized constructs. Thus, vessel and cavity re-endothelialization creates superior vascularized scaffolds for use in whole-organ recellularization applications.
Conflict of interest statement
Figures




Similar articles
-
Improving functional re-endothelialization of acellular liver scaffold using REDV cell-binding domain.Acta Biomater. 2018 Sep 15;78:151-164. doi: 10.1016/j.actbio.2018.07.046. Epub 2018 Jul 31. Acta Biomater. 2018. PMID: 30071351 Free PMC article.
-
An efficient strategy to recellularization of a rat aorta scaffold: an optimized decellularization, detergent removal, and Apelin-13 immobilization.Biomater Res. 2022 Sep 22;26(1):46. doi: 10.1186/s40824-022-00295-1. Biomater Res. 2022. PMID: 36138491 Free PMC article.
-
Tissue Engineered Small Vessel Conduits - The Anti-Thrombotic Effect of Re-Endothelialization of Decellularized Baboon Arteries: A Preliminary Experimental Study.Med Sci Monit Basic Res. 2017 Oct 30;23:344-351. doi: 10.12659/msmbr.905978. Med Sci Monit Basic Res. 2017. PMID: 29081492 Free PMC article.
-
Whole Cardiac Tissue Bioscaffolds.Adv Exp Med Biol. 2018;1098:85-114. doi: 10.1007/978-3-319-97421-7_5. Adv Exp Med Biol. 2018. PMID: 30238367 Review.
-
Hearts beating through decellularized scaffolds: whole-organ engineering for cardiac regeneration and transplantation.Crit Rev Biotechnol. 2016 Aug;36(4):705-15. doi: 10.3109/07388551.2015.1007495. Epub 2015 Mar 5. Crit Rev Biotechnol. 2016. PMID: 25739987 Review.
Cited by
-
Transplantation of bioengineered liver capable of extended function in a preclinical liver failure model.Am J Transplant. 2022 Mar;22(3):731-744. doi: 10.1111/ajt.16928. Epub 2022 Jan 5. Am J Transplant. 2022. PMID: 34932270 Free PMC article.
-
Impact of various detergent-based immersion and perfusion decellularization strategies on the novel caprine pancreas derived extracellular matrix scaffold.Front Bioeng Biotechnol. 2023 Sep 18;11:1253804. doi: 10.3389/fbioe.2023.1253804. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37790257 Free PMC article.
-
Re-epithelialization of whole porcine kidneys with renal epithelial cells.J Tissue Eng. 2017 Jul 3;8:2041731417718809. doi: 10.1177/2041731417718809. eCollection 2017 Jan-Dec. J Tissue Eng. 2017. PMID: 28758007 Free PMC article.
-
The Future of Microsurgery: Vascularized Composite Allotransplantation and Engineering Vascularized Tissue.J Hand Microsurg. 2024 Apr 16;16(1):100011. doi: 10.1055/s-0042-1757182. eCollection 2024 Mar. J Hand Microsurg. 2024. PMID: 38854368 Free PMC article.
-
Hemocompatibility improvement of perfusion-decellularized clinical-scale liver scaffold through heparin immobilization.Sci Rep. 2015 Jun 1;5:10756. doi: 10.1038/srep10756. Sci Rep. 2015. PMID: 26030843 Free PMC article.
References
-
- Butcher JT, Mahler GJ, Hockaday LA (2011) Aortic valve disease and treatment: the need for naturally engineered solutions. Adv Drug Deliv Rev 63: 242–268. - PubMed
-
- Karikkineth BC, Zimmermann WH (2013) Myocardial tissue engineering and heart muscle repair. Curr Pharm Biotechnol 14: 4–11. - PubMed
-
- Gaballa MA, Sunkomat JN, Thai H, Morkin E, Ewy G, et al. (2006) Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant 25: 946–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

