Efficacy of cyclooctadepsipeptides and aminophenylamidines against larval, immature and mature adult stages of a parasitologically characterized trichurosis model in mice
- PMID: 24587460
- PMCID: PMC3930511
- DOI: 10.1371/journal.pntd.0002698
Efficacy of cyclooctadepsipeptides and aminophenylamidines against larval, immature and mature adult stages of a parasitologically characterized trichurosis model in mice
Abstract
Background: The genus Trichuris includes parasites of major relevance in veterinary and human medicine. Despite serious economic losses and enormous impact on public health, treatment options against whipworms are very limited. Additionally, there is an obvious lack of appropriately characterized experimental infection models. Therefore, a detailed parasitological characterization of a Trichuris muris isolate was performed in C57BL/10 mice. Subsequently, the in vivo efficacies of the aminophenylamidines amidantel, deacylated amidantel (dAMD) and tribendimidine as well as the cyclooctadepsipeptides emodepside and in particular PF1022A were analyzed. This was performed using various administration routes and treatment schemes targeting histotropic and further developed larval as well as immature and mature adult stages.
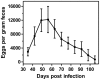
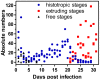
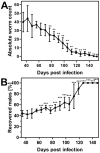
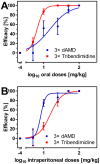
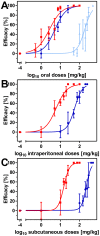
Methodology/principal findings: Duration of prepatent period, time-dependent localization of larvae during period of prepatency as well as the duration of patency of the infection were determined before drugs were tested in the characterized trichurosis model. Amidantel showed no effect against mature adult T. muris. Tribendimidine showed significantly higher potency than dAMD after oral treatments (ED50 values of 6.5 vs. 15.1 mg/kg). However, the opposite was found for intraperitoneal treatments (ED50 values of 15.3 vs. 8.3 mg/kg). When emodepside and PF1022A were compared, the latter was significantly less effective against mature adults following intraperitoneal (ED50 values of 6.1 vs. 55.7 mg/kg) or subcutaneous (ED50 values of 15.2 vs. 225.7 mg/kg) administration. Only minimal differences were observed following oral administration (ED50 values of 2.7 vs. 5.2 mg/kg). Triple and most single oral doses with moderate to high dosages of PF1022A showed complete efficacy against histotropic second stage larvae (3 × 100 mg/kg or 1 × 250 mg/kg), further developed larvae (3 × 10 mg/kg or 1 × 100 mg/kg) and immature adults (3 × 10 mg/kg or 1×100 mg/kg). Histotropic first stage larvae were only eliminated after three doses of PF1022A (3 × 100 mg/kg) but not after a single dose.
Conclusions/significance: These results indicate that the cyclooctadepsipeptides are a drug class with promising candidates for further evaluation for the treatment of trichurosis of humans and livestock animals in single dose regimens.
Conflict of interest statement
Daniel Kulke, PhD student of the Institute of Parasitology and Tropical Veterinary Medicine, Freie Universität Berlin, is employed by Bayer HealthCare, Global Drug Discovery, Animal Health, developing veterinary pharmaceuticals including dewormers. Furthermore, Achim Harder was an employee of Bayer HealthCare when the study was conducted. Except of Achim Harder and Daniel Kulke, Bayer HealthCare was not involved in study design, data collection, data analysis or preparation of the manuscript. The decision to publish the manuscript was jointly taken. This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures

Similar articles
-
Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections.Parasit Vectors. 2019 May 14;12(1):226. doi: 10.1186/s13071-019-3476-x. Parasit Vectors. 2019. PMID: 31088525 Free PMC article.
-
In vitro efficacy of cyclooctadepsipepdtides and aminophenylamidines alone and in combination against third-stage larvae and adult worms of Nippostrongylus brasiliensis and first-stage larvae of Trichinella spiralis.Parasitol Res. 2013 Jan;112(1):335-45. doi: 10.1007/s00436-012-3141-1. Epub 2012 Oct 2. Parasitol Res. 2013. PMID: 23052772
-
In vivo efficacy of PF1022A and nicotinic acetylcholine receptor agonists alone and in combination against Nippostrongylus brasiliensis.Parasitology. 2013 Sep;140(10):1252-65. doi: 10.1017/S0031182013000632. Epub 2013 Jun 7. Parasitology. 2013. PMID: 23742764
-
Cyclooctadepsipeptides--an anthelmintically active class of compounds exhibiting a novel mode of action.Int J Antimicrob Agents. 2003 Sep;22(3):318-31. doi: 10.1016/s0924-8579(03)00219-x. Int J Antimicrob Agents. 2003. PMID: 13678839 Review.
-
Anthelmintic cyclcooctadepsipeptides: complex in structure and mode of action.Trends Parasitol. 2012 Sep;28(9):385-94. doi: 10.1016/j.pt.2012.06.005. Epub 2012 Jul 31. Trends Parasitol. 2012. PMID: 22858281 Review.
Cited by
-
Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections.Parasit Vectors. 2019 May 14;12(1):226. doi: 10.1186/s13071-019-3476-x. Parasit Vectors. 2019. PMID: 31088525 Free PMC article.
-
Population Pharmacokinetics and Exposure-Response Analysis of Tribendimidine To Improve Treatment for Children with Hookworm Infection.Antimicrob Agents Chemother. 2021 Jan 20;65(2):e01778-20. doi: 10.1128/AAC.01778-20. Print 2021 Jan 20. Antimicrob Agents Chemother. 2021. PMID: 33139293 Free PMC article.
-
In Vitro and In Vivo Drug-Drug Interaction Study of the Effects of Ivermectin and Oxantel Pamoate on Tribendimidine.Antimicrob Agents Chemother. 2018 Dec 21;63(1):e00762-18. doi: 10.1128/AAC.00762-18. Print 2019 Jan. Antimicrob Agents Chemother. 2018. PMID: 30323047 Free PMC article.
-
A BRIEF REVIEW ON THE MODE OF ACTION OF ANTINEMATODAL DRUGS.Acta Vet (Beogr). 2017 Jun;67(2):137-152. doi: 10.1515/acve-2017-0013. Epub 2017 Jun 26. Acta Vet (Beogr). 2017. PMID: 29416226 Free PMC article.
-
Characterization of the Ca2+-gated and voltage-dependent K+-channel Slo-1 of nematodes and its interaction with emodepside.PLoS Negl Trop Dis. 2014 Dec 18;8(12):e3401. doi: 10.1371/journal.pntd.0003401. eCollection 2014 Dec. PLoS Negl Trop Dis. 2014. PMID: 25521608 Free PMC article.
References
-
- Knopp S, Steinmann P, Keiser J, Utzinger J (2012) Nematode infections: soil-transmitted helminths and trichinella. Infect Dis Clin North Am 26: 341–358. - PubMed
-
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH (2009) Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373: 1570–1575. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

