Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization
- PMID: 24587574
- PMCID: PMC3920317
- DOI: 10.5665/sleep.3488
Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization
Abstract
Study objectives: Sleep deprivation, or sleep disruption, enhances pain in human subjects. Chronic musculoskeletal pain is prevalent in our society, and constitutes a tremendous public health burden. Although preclinical models of neuropathic and inflammatory pain demonstrate effects on sleep, few studies focus on musculoskeletal pain. We reported elsewhere in this issue of SLEEP that musculoskeletal sensitization alters sleep of mice. In this study we hypothesize that sleep fragmentation during the development of musculoskeletal sensitization will exacerbate subsequent pain responses and alter sleep-wake behavior of mice.
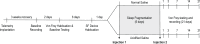
Design: This is a preclinical study using C57BL/6J mice to determine the effect on behavioral outcomes of sleep fragmentation combined with musculoskeletal sensitization.
Methods: Musculoskeletal sensitization, a model of chronic muscle pain, was induced using two unilateral injections of acidified saline (pH 4.0) into the gastrocnemius muscle, spaced 5 days apart. Musculoskeletal sensitization manifests as mechanical hypersensitivity determined by von Frey filament testing at the hindpaws. Sleep fragmentation took place during the consecutive 12-h light periods of the 5 days between intramuscular injections. Electroencephalogram (EEG) and body temperature were recorded from some mice at baseline and for 3 weeks after musculoskeletal sensitization. Mechanical hypersensitivity was determined at preinjection baseline and on days 1, 3, 7, 14, and 21 after sensitization. Two additional experiments were conducted to determine the independent effects of sleep fragmentation or musculoskeletal sensitization on mechanical hypersensitivity.
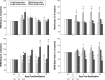
Results: Five days of sleep fragmentation alone did not induce mechanical hypersensitivity, whereas sleep fragmentation combined with musculoskeletal sensitization resulted in prolonged and exacerbated mechanical hypersensitivity. Sleep fragmentation combined with musculoskeletal sensitization had an effect on subsequent sleep of mice as demonstrated by increased numbers of sleep-wake state transitions during the light and dark periods; changes in nonrapid eye movement (NREM) sleep, rapid eye movement sleep, and wakefulness; and altered delta power during NREM sleep. These effects persisted for at least 3 weeks postsensitization.
Conclusions: Our data demonstrate that sleep fragmentation combined with musculoskeletal sensitization exacerbates the physiological and behavioral responses of mice to musculoskeletal sensitization, including mechanical hypersensitivity and sleep-wake behavior. These data contribute to increasing literature demonstrating bidirectional relationships between sleep and pain. The prevalence and incidence of insufficient sleep and pathologies characterized by chronic musculoskeletal pain are increasing in the United States. These demographic data underscore the need for research focused on insufficient sleep and chronic pain so that the quality of life for the millions of individuals with these conditions may be improved.
Keywords: Mice; pain; rodent; sleep restriction; von Frey.
Figures




Similar articles
-
Musculoskeletal sensitization and sleep: chronic muscle pain fragments sleep of mice without altering its duration.Sleep. 2014 Mar 1;37(3):505-13. doi: 10.5665/sleep.3486. Sleep. 2014. PMID: 24587573 Free PMC article.
-
Acute increases in intramuscular inflammatory cytokines are necessary for the development of mechanical hypersensitivity in a mouse model of musculoskeletal sensitization.Brain Behav Immun. 2015 Feb;44:213-20. doi: 10.1016/j.bbi.2014.10.009. Epub 2014 Oct 22. Brain Behav Immun. 2015. PMID: 25449670
-
Behavioral sleep-wake homeostasis and EEG delta power are decoupled by chronic sleep restriction in the rat.Sleep. 2015 May 1;38(5):685-97. doi: 10.5665/sleep.4656. Sleep. 2015. PMID: 25669184 Free PMC article.
-
Central sensitization: implications for the diagnosis and treatment of pain.Pain. 2011 Mar;152(3 Suppl):S2-S15. doi: 10.1016/j.pain.2010.09.030. Epub 2010 Oct 18. Pain. 2011. PMID: 20961685 Free PMC article. Review.
-
[Sleep deprivation and pain: a review of the newest literature].Schmerz. 2014 Apr;28(2):141-6. doi: 10.1007/s00482-014-1394-6. Schmerz. 2014. PMID: 24643753 Review. German.
Cited by
-
Is melatonin the next "new" therapy to improve sleep and reduce pain?Sleep. 2014 Sep 1;37(9):1405-6. doi: 10.5665/sleep.3980. Sleep. 2014. PMID: 25142562 Free PMC article. No abstract available.
-
EEG Radiotelemetry in Small Laboratory Rodents: A Powerful State-of-the Art Approach in Neuropsychiatric, Neurodegenerative, and Epilepsy Research.Neural Plast. 2016;2016:8213878. doi: 10.1155/2016/8213878. Epub 2015 Dec 24. Neural Plast. 2016. PMID: 26819775 Free PMC article. Review.
-
A role for neuroimmune signaling in a rat model of Gulf War Illness-related pain.Brain Behav Immun. 2021 Jan;91:418-428. doi: 10.1016/j.bbi.2020.10.022. Epub 2020 Oct 27. Brain Behav Immun. 2021. PMID: 33127584 Free PMC article.
-
Sleep fragmentation delays wound healing in a mouse model of type 2 diabetes.Sleep. 2018 Nov 1;41(11):zsy156. doi: 10.1093/sleep/zsy156. Sleep. 2018. PMID: 30107617 Free PMC article.
-
Restless legs syndrome and pain disorders: what's in common?Curr Pain Headache Rep. 2014 Nov;18(11):461. doi: 10.1007/s11916-014-0461-0. Curr Pain Headache Rep. 2014. PMID: 25249423 Review.
References
-
- Effect of short sleep duration on daily activities--United States, 2005-2008. MMWR Morb Mortal Wkly Rep. 2011;60:239–42. - PubMed
-
- Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. - PubMed
-
- Opp MR. Sleep and psychoneuroimmunology. Immunol Allergy Clin North Am. 2009;29:295–307. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

