Increased use-dependent plasticity in chronic insomnia
- PMID: 24587576
- PMCID: PMC3920319
- DOI: 10.5665/sleep.3492
Increased use-dependent plasticity in chronic insomnia
Abstract
Study objectives: During normal sleep several neuroplasticity changes occur, some of which are considered to be fundamental to strengthen memories. Given the evidence linking sleep to neuroplasticity, it is conceivable that individuals with chronic sleep disruption, such as patients with chronic insomnia (CI), would experience abnormalities in neuroplastic processes during daytime. Protocols testing use-dependent plasticity (UDP), one of the mechanisms underlying formation of motor memories traces, provide a sensitive measure to assess neuroplasticity in the context of motor training.
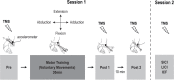
Design and participants: A well-established transcranial magnetic stimulation (TMS) paradigm was used to evaluate the ability of patients with CI and age-matched good sleeper controls to undergo UDP. We also investigated the effect of insomnia on intracortical motor excitability measures reflecting GABAergic and glutamatergic mechanisms.
Setting: Human Brain Physiology Laboratory, Johns Hopkins Medical Institutions.
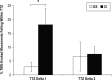
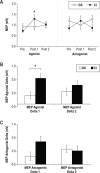
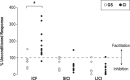
Measurements and results: We found that patients with CI experienced increased UDP changes relative to controls. This effect was not due to differences in motor training. In addition, patients with CI showed enhanced intracortical facilitation relative to controls, in the absence of changes in intracortical inhibitory measures.
Conclusion: This study provides the first evidence that patients with chronic insomnia have an increased plasticity response to physical exercise, possibly due to larger activation of glutamatergic mechanisms. This suggests a heightened state of neuroplasticity, which may reflect a form of maladaptive plasticity, similar to what has been described in dystonia patients and chronic phantom pain after amputation. These results could lead to development of novel treatments for chronic insomnia.
Keywords: Insomnia; memory; motor training; plasticity; transcranial magnetic stimulation.
Figures
References
-
- Stickgold R, Walker MP. Sleep and memory: the ongoing debate. Sleep. 2005;28:1225–7. - PubMed
-
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. - PubMed
-
- Nissen C, Kloepfer C, Feige B, et al. Sleep-related memory consolidation in primary insomnia. J Sleep Res. 2011;20:129–36. - PubMed
-
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

