Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice
- PMID: 24587679
- PMCID: PMC3934475
- DOI: 10.3748/wjg.v20.i8.2051
Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice
Abstract
Aim: To characterize longitudinally the inflammation and the gut microbiota dynamics in a mouse model of dextran sulfate sodium (DSS)-induced colitis.
Methods: In animal models, the most common method used to trigger colitis is based on the oral administration of the sulfated polysaccharides DSS. The murine DSS colitis model has been widely adopted to induce severe acute, chronic or semi-chronic colitis, and has been validated as an important model for the translation of mice data to human inflammatory bowel disease (IBD). However, it is now clear that models characterized by mild intestinal damage are more accurate for studying the effects of therapeutic agents. For this reason, we have developed a murine model of mild colitis to study longitudinally the inflammation and microbiota dynamics during the intestinal repair processes, and to obtain data suitable to support the recovery of gut microbiota-host homeostasis.
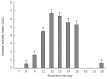
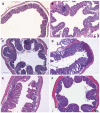
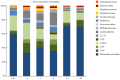
Results: All plasma cytokines evaluated, except IL-17, began to increase (P < 0.05), after 7 d of DSS administration. IL-17 only began to increase 4 d after DSS withdrawal. IL-1β and IL-17 continue to increase during the recovery phase, even when clinical signs of colitis had disappeared. IL-6, IL-10 and IFN-γ reached their maxima 4 d after DSS withdrawal and decreased during the late recovery phase. TNFα reached a peak (a three- fold increase, P < 0.05), after which it slightly decreased, only to increase again close to the end of the recovery phase. DSS administration induced profound and rapid changes in the mice gut microbiota. After 3 d of DSS administration, we observed a major reduction in Bacteroidetes/Prevotella and a corresponding increase in Bacillaceae, with respect to control mice. In particular, Bacteroidetes/Prevotella decreased from a relative abundance of 59.42%-33.05%, while Bacillaceae showed a concomitant increase from 2.77% to 10.52%. Gut microbiota rapidly shifted toward a healthy profile during the recovery phase and returned normal 4 d after DSS withdrawal. Cyclooxygenase 2 expression started to increase 4 d after DSS withdrawal (P < 0.05), when dysbiosis had recovered, and continued to increase during the recovery phase. Taken together, these data indicated that a chronic phase of intestinal inflammation, characterized by the absence of dysbiosis, could be obtained in mice using a single DSS cycle.
Conclusion: Dysbiosis contributes to the local and systemic inflammation that occurs in the DSS model of colitis; however, chronic bowel inflammation is maintained even after recovery from dysbiosis.
Keywords: Colitis, Dysbiosis; Cyclooxygenase 2; Dextran sulfate sodium; Inflammation.
Figures




References
-
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. - PubMed
-
- Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, Michaëlsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–844. - PubMed
-
- Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49:9–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

