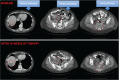
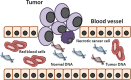
Tumor heterogeneity in the clinic: is it a real problem?
- PMID: 24587830
- PMCID: PMC3932055
- DOI: 10.1177/1758834013517414
Tumor heterogeneity in the clinic: is it a real problem?
Abstract
Tumor heterogeneity is one of the major problems limiting the efficacy of targeted therapies and compromising treatment outcomes. A better understanding of tumor biology has advanced our knowledge of the molecular landscape of cancer to an unprecedented level. However, most patients with advanced cancers treated with appropriately selected targeted therapies become resistant to the therapy, ultimately developing disease progression and succumbing to metastatic disease. Multiple factors account for therapeutic failures, which include cancer cells accumulating new molecular aberrations as a consequence of tumor progression and selection pressure of cancer therapies. Therefore, single agent targeted therapies, often administered in advanced stages, are unlikely to have a sufficiently lethal effect in most cancers. Finally, the molecular profile of cancer can change over time, which we are not able to monitor with existing strategies using tumor tissue biopsies as the gold standard for molecular diagnostics. Novel technologies focusing on testing low-risk, easily obtainable material, such as molecular cell-free DNA from plasma, can fill that gap and allow personalized therapy to be delivered in real time.
Keywords: molecular aberrations; targeted therapies; tumor heterogeneity.
Conflict of interest statement
Figures
References
-
- Amado R., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D., et al. (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26: 1626–1634 - PubMed
-
- Braun S., Pantel K., Muller P., Janni W., Hepp F., Kentenich C., et al. (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342: 525–533 - PubMed
-
- Cho K., Vogelstein B. (1992) Genetic alterations in the adenoma–carcinoma sequence. Cancer 70: 1727–1731 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous