The effects of inhaled albuterol in transient tachypnea of the newborn
- PMID: 24587948
- PMCID: PMC3936040
- DOI: 10.4168/aair.2014.6.2.126
The effects of inhaled albuterol in transient tachypnea of the newborn
Abstract
Purpose: Transient tachypnea of the newborn (TTN) is a disorder caused by the delayed clearance of fetal alveolar fluid. β-adrenergic agonists such as albuterol (salbutamol) are known to catalyze lung fluid absorption. This study examined whether inhalational salbutamol therapy could improve clinical symptoms in TTN. Additional endpoints included the diagnostic and therapeutic efficacy of salbutamol as well as its overall safety.
Methods: From January 2010 through December 2010, we conducted a prospective study of 40 newborns hospitalized with TTN in the neonatal intensive care unit. Patients were given either inhalational salbutamol (28 patients) or placebo (12 patients), and clinical indices were compared.
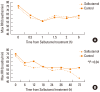
Results: The duration of tachypnea was shorter in patients receiving inhalational salbutamol therapy, although this difference was not statistically significant. The duration of supplemental oxygen therapy and the duration of empiric antibiotic treatment were significantly shorter in the salbutamol-treated group. No adverse effects were observed in either treatment group.
Conclusions: Inhalational salbutamol therapy reduced the duration of supplemental oxygen therapy and the duration of empiric antibiotic treatment, with no adverse effects. However, the time between salbutamol therapy and clinical improvement was too long to allow definitive conclusions to be drawn. Further studies examining a larger number of patients with strict control over dosage and frequency of salbutamol inhalations are necessary to better direct the treatment of TTN.
Keywords: Transient tachypnea of the newborn; albuterol; inhalation.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures
References
-
- Abu-Shaweesh JM. Respiratory disorders in preterm and term infants. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin's neonatal-perinatal medicine: diseases of the fetus and infant. 9th ed. St. Louis (MO): Elsevier Mosby; 2010. pp. 1162–1163.
-
- Clark RH. The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol. 2005;25:251–257. - PubMed
-
- Takaya A, Igarashi M, Nakajima M, Miyake H, Shima Y, Suzuki S. Risk factors for transient tachypnea of the newborn in infants delivered vaginally at 37 weeks or later. J Nippon Med Sch. 2008;75:269–273. - PubMed
-
- Lewis V, Whitelaw A. Furosemide for transient tachypnea of the newborn. Cochrane Database Syst Rev. 2002;(1):CD003064. - PubMed
-
- Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol. 2006;30:34–43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

