Tricarballylic ester formation during biosynthesis of fumonisin mycotoxins in Fusarium verticillioides
- PMID: 24587959
- PMCID: PMC3933019
- DOI: 10.1080/21501203.2013.874540
Tricarballylic ester formation during biosynthesis of fumonisin mycotoxins in Fusarium verticillioides
Abstract
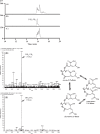
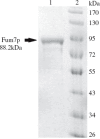
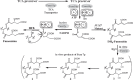
Fumonisins are agriculturally important mycotoxins produced by the maize pathogen Fusarium verticillioides. The chemical structure of fumonisins contains two tricarballylic esters, which are rare structural moieties and important for toxicity. The mechanism for the tricarballylic ester formation is not well understood. FUM7 gene of F. verticillioides was predicted to encode a dehydrogenase/reductase, and when it was deleted, the mutant produced tetradehydro fumonisins (DH4-FB). MS and NMR analysis of DH4-FB1 indicated that the esters consist of aconitate with a 3'-alkene function, rather than a 2'-alkene function. Interestingly, the purified DH4-FB1 eventually yielded three chromatographic peaks in HPLC. However, MS revealed that the metabolites of the three peaks all had the same mass as the initial single-peak DH4-FB1. The results suggest that DH4-FB1 can undergo spontaneous isomerization, probably including both cis-trans stereoisomerization and 3'- to 2'-ene regioisomerization. In addition, when FUM7 was expressed in Escherichia coli and the resulting enzyme, Fum7p, was incubated with DH4-FB, no fumonisin with typical tricarballylic esters was formed. Instead, new fumonisin analogs that probably contained isocitrate and/or oxalosuccinate esters were formed, which reveals new insight into fumonisin biosynthesis. Together, the data provided both genetic and biochemical evidence for the mechanism of tricarballylic ester formation in fumonisin biosynthesis.
Keywords: Fusarium verticillioides; biosynthesis; fumonisins; mycotoxins.
Figures


Similar articles
-
Deletion analysis of FUM genes involved in tricarballylic ester formation during fumonisin biosynthesis.J Agric Food Chem. 2006 Dec 13;54(25):9398-404. doi: 10.1021/jf0617869. J Agric Food Chem. 2006. PMID: 17147424
-
A bidomain nonribosomal peptide synthetase encoded by FUM14 catalyzes the formation of tricarballylic esters in the biosynthesis of fumonisins.Biochemistry. 2006 Feb 28;45(8):2561-9. doi: 10.1021/bi052085s. Biochemistry. 2006. PMID: 16489749
-
Evaluating three commonly used growth media for assessing fumonisin analogues FB1, FB2 and FB3 production by nine Fusarium verticillioides isolates.Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017 Feb;34(2):291-298. doi: 10.1080/19440049.2016.1266397. Epub 2016 Dec 14. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017. PMID: 27899061
-
NMR structural studies of fumonisin B1 and related compounds from Fusarium moniliforme.Adv Exp Med Biol. 1996;392:75-91. doi: 10.1007/978-1-4899-1379-1_7. Adv Exp Med Biol. 1996. PMID: 8850607 Review.
-
[Molecular genetics on fumonisin-producing strains of Fusarium verticillioides].Wei Sheng Yan Jiu. 2005 Mar;34(2):248-51. Wei Sheng Yan Jiu. 2005. PMID: 15952676 Review. Chinese.
Cited by
-
Branching and converging pathways in fungal natural product biosynthesis.Fungal Biol Biotechnol. 2022 Mar 7;9(1):6. doi: 10.1186/s40694-022-00135-w. Fungal Biol Biotechnol. 2022. PMID: 35255990 Free PMC article. Review.
-
The palmitoyl-CoA ligase Fum16 is part of a Fusarium verticillioides fumonisin subcluster involved in self-protection.mBio. 2025 Feb 5;16(2):e0268124. doi: 10.1128/mbio.02681-24. Epub 2024 Dec 20. mBio. 2025. PMID: 39704544 Free PMC article.
-
Current Insights in Fungal Importance-A Comprehensive Review.Microorganisms. 2023 May 24;11(6):1384. doi: 10.3390/microorganisms11061384. Microorganisms. 2023. PMID: 37374886 Free PMC article. Review.
-
Discovery of the actinoplanic acid pathway in Streptomyces rapamycinicus reveals a genetically conserved synergism with rapamycin.J Biol Chem. 2018 Dec 28;293(52):19982-19995. doi: 10.1074/jbc.RA118.005314. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327433 Free PMC article.
-
FunOrder: A robust and semi-automated method for the identification of essential biosynthetic genes through computational molecular co-evolution.PLoS Comput Biol. 2021 Sep 27;17(9):e1009372. doi: 10.1371/journal.pcbi.1009372. eCollection 2021 Sep. PLoS Comput Biol. 2021. PMID: 34570757 Free PMC article.
References
-
- Bezuidenhout SC, Gelderblom WCA, Gorst-Allman CP, Horak RM, Marasas WFO, Spiteller G, Vleggaar R. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem Commun. 1988;11:743–745.
-
- Blackwell BA, Edwards OE, Fruchier A, ApSimon JW, Miller JD. NMR structural studies of fumonisin B1 and related compounds from Fusarium moniliforme. Adv Exp Med Biol. 1996;392:75–91. - PubMed
-
- Bojja RS, Cerny RL, Proctor RH, Du L. Determining the biosynthetic sequence in the early steps of the fumonisin pathway by use of three gene-disruption mutants of Fusarium verticillioides. J Agric Food Chem. 2004;52:2855–2860. - PubMed
-
- Branham BE, Plattner RD. Alanine is a precursor in the biosynthesis of fumonisin B1 by Fusarium moniliforme. Mycopathologia. 1993;124:99–104. - PubMed
-
- Butchko RA, Plattner RD, Proctor RH. FUM13 encodes a short chain dehydrogenase/reductase required for C-3 carbonyl reduction during fumonisin biosynthesis in Gibberella moniliformis. J Agric Food Chem. 2003;51:3000–3006. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
