Eradication of Human Ovarian Cancer Cells by Transgenic Expression of Recombinant DNASE1, DNASE1L3, DNASE2, and DFFB Controlled by EGFR Promoter: Novel Strategy for Targeted Therapy of Cancer
- PMID: 24587967
- PMCID: PMC3938193
- DOI: 10.4172/2157-7412.1000152
Eradication of Human Ovarian Cancer Cells by Transgenic Expression of Recombinant DNASE1, DNASE1L3, DNASE2, and DFFB Controlled by EGFR Promoter: Novel Strategy for Targeted Therapy of Cancer
Abstract
Introduction: Ovarian cancer is the most deadly among all gynecological cancers. Patients undergoing systemic therapies of advanced ovarian cancers suffer from horrendous side effects. Cancer survivors and their offspring suffer from iatrogenic consequences of systemic therapies: genetic mutations. The ultimate goal of our work is development of therapies, which selectively and completely eliminate cancer cells, but do not harm healthy cells. An important consideration for attaining this goal is the fact that ovarian cancer cells over-express EGFR or its mutants, what becomes the factor discriminating them from healthy cells - a potential facilitator of personalized therapy.
Specific aim: The specific aim of this project was threefold: (1) to bioengineer suicide genes' carrying vectors guided by synthetic antibodies for EGFRvIII and EGFR; (2) to genetically engineer DNA constructs for the human, recombinant DNASE1, DNASE1L3, DNASE2, and DFFB controlled by the EGFR promoter; (3) to selectively eradicate ovarian cancer cells by intranuclear targeting of the transgenically expressed recombinant DNases.
Methods: Synthetic antibodies for EGFR and EGFRvIII were selected from the human library and used to bioengineer biotag-guided transgenes' vectors. Coding sequences for the human DNASE1, DNASE1L3, DNASE2, DFFB controlled by the EGFR promoter were amplified from the human cDNA and genetically engineered into the plasmid constructs also coding for the fusions with NLS and GFP. The vectors carrying transgenes for the DNases were delivered in vitro into human ovarian cancer cells from ascites and cultures.
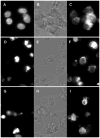
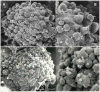
Results: Synthetic antibody guided vectors delivered the transgenes for the recombinant DNases efficiently into the ovarian cancer cells. Transgenic expression and nuclear targeting of the DNases in those cells resulted in destruction of their genomes and led to their death, as validated by labeling with the molecular death tags. In healthy cells, which did not over-express EGFR, no changes were recorded.
Conclusion: Targeted expression of the recombinant DNASE1, DNASE1L3, DNASE2, DFFB in the ovarian cancers in vitro resulted in their complete eradication, but had no effects upon the healthy cells. This novel therapeutic strategy has a potential for streamlining it into in vivo trials, as personalized, targeted therapy of ovarian and other cancers.
Keywords: Apoptosis; Cancer of the ovary; DNase; Epidermal growth factor receptor; Nuclear localization signal; Personalized therapy of cancer; Suicide gene therapy of cancer.
Conflict of interest statement
The authors state no conflict of interest. Marek Malecki MD PhD – Inventor owns the IP for the synthetic genes of his design, transcripts, and expression products used in this work, as well as their streamlining to diagnosis and therapy, all protected by USPTO and WIPO.
Figures



Similar articles
-
Safeguarding Stem Cell-Based Regenerative Therapy against Iatrogenic Cancerogenesis: Transgenic Expression of DNASE1, DNASE1L3, DNASE2, DFFB Controlled By POLA1 Promoter in Proliferating and Directed Differentiation Resisting Human Autologous Pluripotent Induced Stem Cells Leads to their Death.J Stem Cell Res Ther. 2013 Jul 22;Suppl 9(5):21559. doi: 10.4172/2157-7633.S9-005. J Stem Cell Res Ther. 2013. PMID: 25045589 Free PMC article.
-
Targeting NETs using dual-active DNase1 variants.Front Immunol. 2023 May 23;14:1181761. doi: 10.3389/fimmu.2023.1181761. eCollection 2023. Front Immunol. 2023. PMID: 37287977 Free PMC article.
-
Stem cells' guided gene therapy of cancer: New frontier in personalized and targeted therapy.J Cancer Res Ther (Manch). 2014;2(1):22-33. doi: 10.14312/2052-4994.2014-4. J Cancer Res Ther (Manch). 2014. PMID: 24860662 Free PMC article.
-
Dnases in health and disease.Dev Biol. 2017 Sep 1;429(1):1-11. doi: 10.1016/j.ydbio.2017.06.028. Epub 2017 Jun 28. Dev Biol. 2017. PMID: 28666955 Free PMC article. Review.
-
The Nexus of cfDNA and Nuclease Biology.Trends Genet. 2021 Aug;37(8):758-770. doi: 10.1016/j.tig.2021.04.005. Epub 2021 May 15. Trends Genet. 2021. PMID: 34006390 Review.
Cited by
-
DNASE1L3 as an indicator of favorable survival in hepatocellular carcinoma patients following resection.Aging (Albany NY). 2020 Jan 24;12(2):1171-1185. doi: 10.18632/aging.102675. Epub 2020 Jan 24. Aging (Albany NY). 2020. PMID: 31977318 Free PMC article.
-
DNASE1L3 arrests tumor angiogenesis by impairing the senescence-associated secretory phenotype in response to stress.Aging (Albany NY). 2021 Mar 19;13(7):9874-9899. doi: 10.18632/aging.202740. Epub 2021 Mar 19. Aging (Albany NY). 2021. PMID: 33744849 Free PMC article.
-
Comprehensive Analysis Reveals Deoxyribonuclease 1 as a Potential Prognostic and Diagnostic Biomarker in Human Cancers.Cureus. 2024 Mar 14;16(3):e56171. doi: 10.7759/cureus.56171. eCollection 2024 Mar. Cureus. 2024. PMID: 38618458 Free PMC article.
-
Safeguarding Stem Cell-Based Regenerative Therapy against Iatrogenic Cancerogenesis: Transgenic Expression of DNASE1, DNASE1L3, DNASE2, DFFB Controlled By POLA1 Promoter in Proliferating and Directed Differentiation Resisting Human Autologous Pluripotent Induced Stem Cells Leads to their Death.J Stem Cell Res Ther. 2013 Jul 22;Suppl 9(5):21559. doi: 10.4172/2157-7633.S9-005. J Stem Cell Res Ther. 2013. PMID: 25045589 Free PMC article.
-
Killing of cancer cells through the use of eukaryotic expression vectors harbouring genes encoding nucleases and ribonuclease inhibitor.Tumour Biol. 2015 May;36(5):3147-57. doi: 10.1007/s13277-015-3360-z. Epub 2015 Apr 1. Tumour Biol. 2015. PMID: 25874497 Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–432. - PubMed
-
- Chobanian N, Dietrich CS., 3rd Ovarian cancer. Surg Clin North Am. 2008;88:285–299. vi. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
