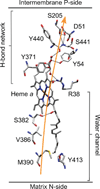
Copper active sites in biology
- PMID: 24588098
- PMCID: PMC4040215
- DOI: 10.1021/cr400327t
Copper active sites in biology
Figures
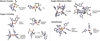




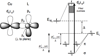

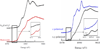




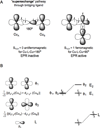



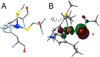



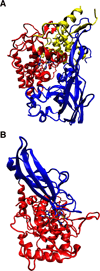


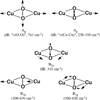
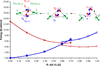
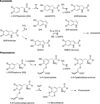
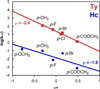
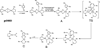
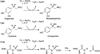
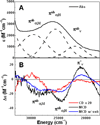
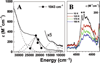
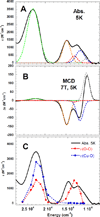
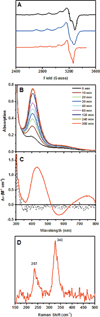
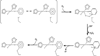
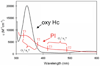
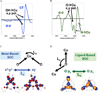
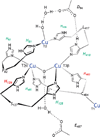
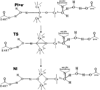
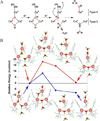
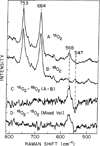
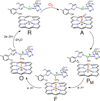
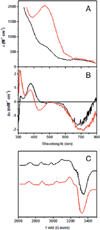
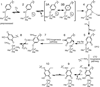
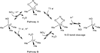
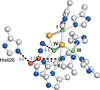
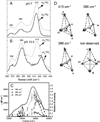
 ) and met-Hc (—), circular dichroism spectrum of oxy-Hc (
) and met-Hc (—), circular dichroism spectrum of oxy-Hc ( ) (15K, acetate pH 5.0), B) Resonance Raman excitation profile of 765 cm−1 (○) and 287 cm−1(●) vibrational features of oxy-Hc superimposed over its absorption spectrum.
) (15K, acetate pH 5.0), B) Resonance Raman excitation profile of 765 cm−1 (○) and 287 cm−1(●) vibrational features of oxy-Hc superimposed over its absorption spectrum.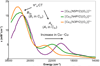

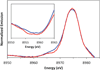
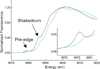
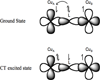
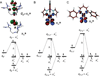
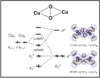
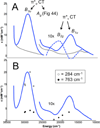
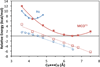
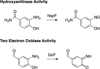
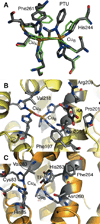
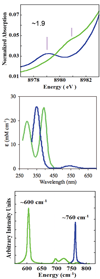
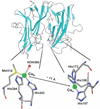
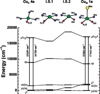
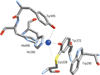
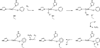
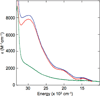
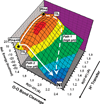
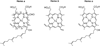
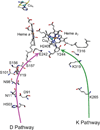
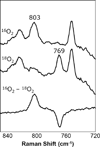
 ) and Busycon canaliculatum (
) and Busycon canaliculatum ( ) oxy-Hc; B: Fourier transform EXAFS of deoxy-Hc (
) oxy-Hc; B: Fourier transform EXAFS of deoxy-Hc ( ) and oxy-Hc (
) and oxy-Hc ( ) from Cancer Irroratus; C: Fourier transform EXAFS of deoxy-Hc (
) from Cancer Irroratus; C: Fourier transform EXAFS of deoxy-Hc ( ) and oxy-Hc (
) and oxy-Hc ( ) from Busycon canaliculatum.
) from Busycon canaliculatum.


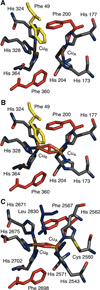
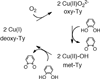
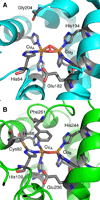
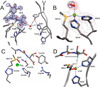
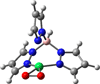
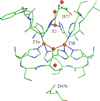
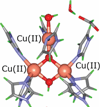
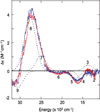
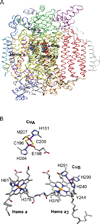
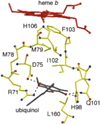
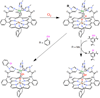
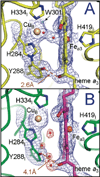
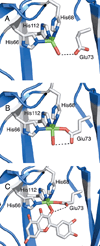
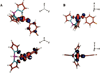
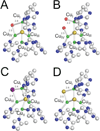
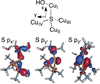
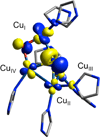
 ) and Cancer (
) and Cancer ( ) met-Hc with azide bound.
) met-Hc with azide bound.
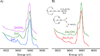
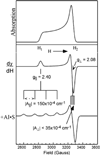
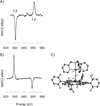
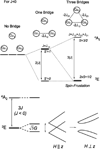
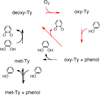
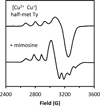
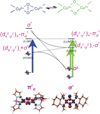
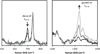
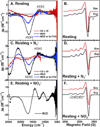
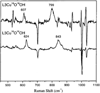
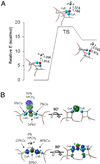
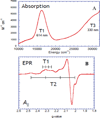
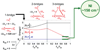
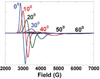
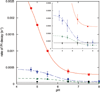
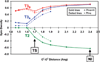
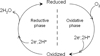
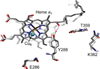
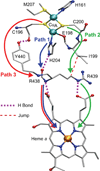
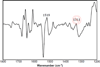
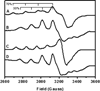
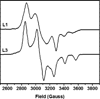
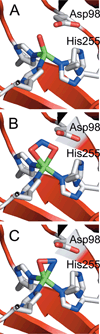
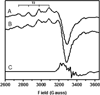
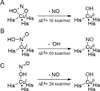
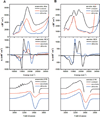
 ) and N3− bound half-met-Hc (
) and N3− bound half-met-Hc ( ); B: EPR spectra of NO2− bound half-met-Hc (
); B: EPR spectra of NO2− bound half-met-Hc ( ) and N3− bound half-met-Hc (
) and N3− bound half-met-Hc ( ).
).

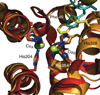

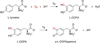





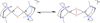

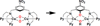







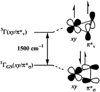

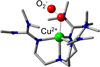


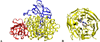
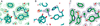




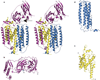
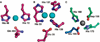








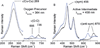


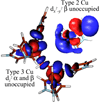





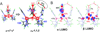








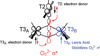




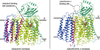








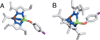






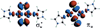





References
-
- Randall DW, Gamelin DR, LaCroix LB, Solomon EI. JBIC. 2000;5:16. - PubMed
-
- Solomon EI, Szilagyi RK, DeBeer George S, Basumallick L. Chem. Rev. 2004;104:419. - PubMed
-
- Lu Y. In: Biocoordination Chemistry, Comp. Chem. II: From Biology to Nanotech. McCleverty JA, Meyer TJ, Que L, Tolman WB, editors. Vol. 8. Oxford: Elsevier; 2004. pp. 91–122.
-
- Solomon EI, Hadt RG. Coordination Chemistry Reviews. 2011;255:774.
-
- Sawyer DT. Oxygen Chemistry. Oxford: Oxford University Press; 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

