Fluorescent sensors for measuring metal ions in living systems
- PMID: 24588137
- PMCID: PMC4096685
- DOI: 10.1021/cr400546e
Fluorescent sensors for measuring metal ions in living systems
Figures

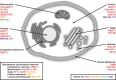

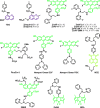



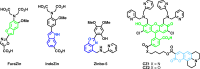



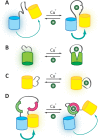


References
-
- Biological Inorganic Chemistry, 1st ed.; Bertini I., Gray H. B., Stiefel E. I., Valentine J. S., Eds.; University Science Books: Sausalito, CA, 2007.
-
- Gladyshev V. N.; Zhang Y. Met. Ions Life Sci. 2013, 12, 529. - PubMed
-
- Cvetkovic A.; Menon A. L.; Thorgersen M. P.; Scott J. W.; Poole F. L. II; Jenney F. E. Jr.; Lancaster W. A.; Praissman J. L.; Shanmukh S.; Vaccaro B. J.; Trauger S. A.; Kalisiak E.; Apon J. V.; Siuzdak G.; Yannone S. M.; Tainer J. A.; Adams M. W. Nature 2010, 466, 779. - PubMed
- Yannone S. M.; Hartung S.; Menon A. L.; Adams M. W.; Tainer J. A. Curr. Opin. Biotechnol. 2012, 23, 89. - PMC - PubMed
-
- Cerchiaro G.; Manieri T. M.; Bertuchi F. R.. Metallomics 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

