Alanine analogues of [D-Trp]CJ-15,208: novel opioid activity profiles and prevention of drug- and stress-induced reinstatement of cocaine-seeking behaviour
- PMID: 24588614
- PMCID: PMC4080975
- DOI: 10.1111/bph.12664
Alanine analogues of [D-Trp]CJ-15,208: novel opioid activity profiles and prevention of drug- and stress-induced reinstatement of cocaine-seeking behaviour
Abstract
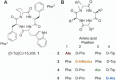
Background and purpose: The novel macrocyclic peptide cyclo[Phe-D-Pro-Phe-D-Trp] ([D-Trp]CJ-15,208) exhibits κ opioid (KOP) receptor antagonist activity in both in vitro and in vivo assays. The four alanine analogues of this peptide were synthesized and characterized both in vitro and in vivo to assess the contribution of different amino acid residues to the activity of [D-Trp]CJ-15,208.
Experimental approach: The peptides were synthesized by a combination of solid phase peptide synthesis and cyclization in solution. The analogues were evaluated in vitro in receptor binding and functional assays, and in vivo with mice using a tail-withdrawal assay for antinociceptive and opioid antagonist activity. Mice demonstrating extinction of cocaine conditioned-place preference (CPP) were pretreated with selected analogues to evaluate prevention of stress or cocaine-induced reinstatement of CPP.
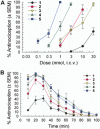
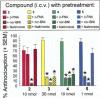
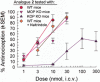
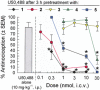
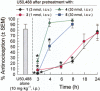
Key results: The alanine analogues displayed pharmacological profiles in vivo distinctly different from [D-Trp]CJ-15,208. While the analogues exhibited varying opioid receptor affinities and κ and μ opioid receptor antagonist activity in vitro, they produced potent opioid receptor-mediated antinociception (ED50 = 0.28-4.19 nmol, i.c.v.) in vivo. Three of the analogues also displayed KOP receptor antagonist activity in vivo. Pretreatment with an analogue exhibiting both KOP receptor agonist and antagonist activity in vivo prevented both cocaine- and stress-induced reinstatement of cocaine-seeking behaviour in the CPP assay in a time-dependent manner.
Conclusions and implications: These unusual macrocyclic peptides exhibit in vivo opioid activity profiles different from the parent compound and represent novel compounds for potential development as therapeutics for drug abuse and possibly as analgesics.
Keywords: CJ-15,208; analgesic; cocaine reinstatement; macrocyclic peptide; κ opioid receptor antagonist.
© 2014 The British Pharmacological Society.
Figures


Similar articles
-
Phenylalanine Stereoisomers of CJ-15,208 and [d-Trp]CJ-15,208 Exhibit Distinctly Different Opioid Activity Profiles.Molecules. 2020 Sep 2;25(17):3999. doi: 10.3390/molecules25173999. Molecules. 2020. PMID: 32887303 Free PMC article.
-
Peptide Kappa Opioid Receptor Ligands and Their Potential for Drug Development.Handb Exp Pharmacol. 2022;271:197-220. doi: 10.1007/164_2021_519. Handb Exp Pharmacol. 2022. PMID: 34463847 Review.
-
Novel opioid cyclic tetrapeptides: Trp isomers of CJ-15,208 exhibit distinct opioid receptor agonism and short-acting κ opioid receptor antagonism.Br J Pharmacol. 2012 Feb;165(4b):1097-108. doi: 10.1111/j.1476-5381.2011.01544.x. Br J Pharmacol. 2012. PMID: 21671905 Free PMC article.
-
The macrocyclic tetrapeptide [D-Trp]CJ-15,208 produces short-acting κ opioid receptor antagonism in the CNS after oral administration.Br J Pharmacol. 2013 May;169(2):426-36. doi: 10.1111/bph.12132. Br J Pharmacol. 2013. PMID: 23425081 Free PMC article.
-
Mixed κ/μ partial opioid agonists as potential treatments for cocaine dependence.Adv Pharmacol. 2014;69:387-418. doi: 10.1016/B978-0-12-420118-7.00010-X. Adv Pharmacol. 2014. PMID: 24484983 Review.
Cited by
-
Phenylalanine Stereoisomers of CJ-15,208 and [d-Trp]CJ-15,208 Exhibit Distinctly Different Opioid Activity Profiles.Molecules. 2020 Sep 2;25(17):3999. doi: 10.3390/molecules25173999. Molecules. 2020. PMID: 32887303 Free PMC article.
-
Peptide Kappa Opioid Receptor Ligands and Their Potential for Drug Development.Handb Exp Pharmacol. 2022;271:197-220. doi: 10.1007/164_2021_519. Handb Exp Pharmacol. 2022. PMID: 34463847 Review.
-
Identification of c[D-Trp-Phe-β-Ala-β-Ala], the First κ-Opioid Receptor-Specific Negative Allosteric Modulator.ACS Pharmacol Transl Sci. 2024 Sep 11;7(10):3192-3204. doi: 10.1021/acsptsci.4c00372. eCollection 2024 Oct 11. ACS Pharmacol Transl Sci. 2024. PMID: 39416958
-
An analog of [d-Trp]CJ-15,208 exhibits kappa opioid receptor antagonism following oral administration and prevents stress-induced reinstatement of extinguished morphine conditioned place preference.Pharmacol Biochem Behav. 2022 Jun;217:173405. doi: 10.1016/j.pbb.2022.173405. Epub 2022 May 15. Pharmacol Biochem Behav. 2022. PMID: 35584724 Free PMC article.
-
Estrous cycle and HIV-1 Tat protein influence cocaine-conditioned place preference and induced locomotion of female mice.Curr HIV Res. 2014;12(6):388-96. doi: 10.2174/1570162x13666150121105221. Curr HIV Res. 2014. PMID: 25613137 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

