An arranged marriage for precision medicine: hypoxia and genomic assays in localized prostate cancer radiotherapy
- PMID: 24588670
- PMCID: PMC4064607
- DOI: 10.1259/bjr.20130753
An arranged marriage for precision medicine: hypoxia and genomic assays in localized prostate cancer radiotherapy
Abstract
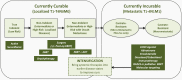
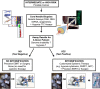
Prostate cancer (CaP) is the most commonly diagnosed malignancy in males in the Western world with one in six males diagnosed in their lifetime. Current clinical prognostication groupings use pathologic Gleason score, pre-treatment prostatic-specific antigen and Union for International Cancer Control-TNM staging to place patients with localized CaP into low-, intermediate- and high-risk categories. These categories represent an increasing risk of biochemical failure and CaP-specific mortality rates, they also reflect the need for increasing treatment intensity and justification for increased side effects. In this article, we point out that 30-50% of patients will still fail image-guided radiotherapy or surgery despite the judicious use of clinical risk categories owing to interpatient heterogeneity in treatment response. To improve treatment individualization, better predictors of prognosis and radiotherapy treatment response are needed to triage patients to bespoke and intensified CaP treatment protocols. These should include the use of pre-treatment genomic tests based on DNA or RNA indices and/or assays that reflect cancer metabolism, such as hypoxia assays, to define patient-specific CaP progression and aggression. More importantly, it is argued that these novel prognostic assays could be even more useful if combined together to drive forward precision cancer medicine for localized CaP.
Figures
Comment in
-
Pre-radiotherapy identification of individual genomic profile to avoid, by resort to customized radiosensitizers, the risk of radioresistance development in patients with localized prostate cancer.Br J Radiol. 2015 Jan;88(1045):20140630. doi: 10.1259/bjr.20140630. Br J Radiol. 2015. PMID: 25363844 Free PMC article. No abstract available.
-
Pre-radiotherapy identification of individual genomic profile to avoid, by resort to customized radiosensitizers, the risk of radioresistance development in patients with localized prostate cancer: author reply.Br J Radiol. 2015 Jan;88(1045):20140701. doi: 10.1259/bjr.20140701. Br J Radiol. 2015. PMID: 25363875 Free PMC article. No abstract available.
Similar articles
-
Array CGH as a potential predictor of radiocurability in intermediate risk prostate cancer.Acta Oncol. 2010 Oct;49(7):888-94. doi: 10.3109/0284186X.2010.499371. Acta Oncol. 2010. PMID: 20590366 Review.
-
Genomic, pathological, and clinical heterogeneity as drivers of personalized medicine in prostate cancer.Urol Oncol. 2015 Feb;33(2):85-94. doi: 10.1016/j.urolonc.2013.10.020. Epub 2014 Apr 24. Urol Oncol. 2015. PMID: 24768356 Review.
-
NKX3.1 haploinsufficiency is prognostic for prostate cancer relapse following surgery or image-guided radiotherapy.Clin Cancer Res. 2012 Jan 1;18(1):308-16. doi: 10.1158/1078-0432.CCR-11-2147. Epub 2011 Nov 2. Clin Cancer Res. 2012. PMID: 22048240
-
Relationship between percent positive biopsies and biochemical outcome after permanent interstitial brachytherapy for clinically organ-confined carcinoma of the prostate gland.Int J Radiat Oncol Biol Phys. 2002 Mar 1;52(3):664-73. doi: 10.1016/s0360-3016(01)02670-0. Int J Radiat Oncol Biol Phys. 2002. PMID: 11849788
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
Cited by
-
Prediction of prostate tumour hypoxia using pre-treatment MRI-derived radiomics: preliminary findings.Radiol Med. 2023 Jun;128(6):765-774. doi: 10.1007/s11547-023-01644-3. Epub 2023 May 17. Radiol Med. 2023. PMID: 37198374 Free PMC article.
-
The changing paradigm of tumour response to irradiation.Br J Radiol. 2017 Jan;90(1069):20160474. doi: 10.1259/bjr.20160474. Epub 2016 Aug 2. Br J Radiol. 2017. PMID: 27416998 Free PMC article. Review.
-
Predicting Treatment Outcomes: The Case for Hypoxia Gene Signatures.EBioMedicine. 2018 Jun;32:3-4. doi: 10.1016/j.ebiom.2018.05.016. EBioMedicine. 2018. PMID: 29776747 Free PMC article. No abstract available.
-
HIF1 and ID1 Interplay Confers Adaptive Survival to HIF1α-Inhibition.Front Cell Dev Biol. 2021 Nov 8;9:724059. doi: 10.3389/fcell.2021.724059. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34820369 Free PMC article.
-
Implementation of the Chick Chorioallantoic Membrane (CAM) Model in Radiation Biology and Experimental Radiation Oncology Research.Cancers (Basel). 2019 Oct 7;11(10):1499. doi: 10.3390/cancers11101499. Cancers (Basel). 2019. PMID: 31591362 Free PMC article. Review.
References
-
- D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol 2003; 21: 2163–72. - PubMed
-
- Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. . Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 2012; 110: E431–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

