Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa
- PMID: 24588809
- PMCID: PMC3973989
- DOI: 10.1186/1471-2180-14-53
Development and antimicrobial susceptibility studies of in vitro monomicrobial and polymicrobial biofilm models with Aspergillus fumigatus and Pseudomonas aeruginosa
Abstract
Background: Mixed microbial infections of the respiratory tracts with P. aeruginosa and A. fumigatus capable of producing biofilms are commonly found in cystic fibrosis patients. The primary objective of this study was to develop an in vitro model for P. aeruginosa and A. fumigatus polymicrobial biofilm to study the efficacy of various antimicrobial drugs alone and in combinations against biofilm-embedded cells. Simultaneous static cocultures of P. aeruginosa and sporelings were used for the development of in vitro P. aeruginosa-A. fumigatus polymicrobial biofilm in SD broth in 24-well cell culture plates at 35°C, and the biofilm formation was monitored microscopically and spectrophotometrically. Using P. aeruginosa-A. fumigatus sporelings cocultures we examined the effects of various antimicrobial drugs alone and in combination against polymicrobial biofilm by CFU and tetrazolium reduction assays.
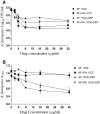
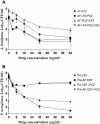
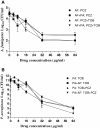
Results: In simultaneous static cocultures P. aeruginosa cells killed A. fumigatus conidia, whereas the bacterial cells showed no substantial fungicidal effect on sporelings grown for 12 h or longer at 35°C. Monospecies cultures of P. aeruginosa produced loosely adhered monomicrobial biofilm and addition of 10% bovine serum to the growth medium inhibited the formation of monomicrobial biofilm by P. aeruginosa whereas it produced tightly adhered polymicrobial biofilm in the presence of A. fumigatus mycelial growth. A. fumigatus produced firmly adherent monomicrobial and polymicrobial biofilms. A comparison of CFU and MTT assays showed that the latter is unsuitable for studying the effectiveness of antimicrobial treatment against polymicrobial biofilm. Tobramycin alone and in combination with posaconazole was highly effective against monomicrobial and polymicrobial biofilms of P. aeruginosa whereas cefepime alone and in combination with posaconazole showed excellent activity against monomicrobial biofilm of P. aeruginosa but was less effective against polymicrobial biofilm. Monomicrobial and polymicrobial biofilms of A. fumigatus showed similar susceptibility to posaconazole with and without the antibacterial drug.
Conclusions: Simultaneous static coculture of A. fumigatus sporelings grown for 12 h or longer was superior to ungerminated conidia with P. aeruginosa for the development of A. fumigatus-P. aeruginosa biofilm. P. aeruginosa-A. fumigatus polymicrobial biofilm shows differential susceptibility to antimicrobial drugs whereas the susceptibility of A. fumigatus to antimicrobial drugs was unchanged.
Figures



Similar articles
-
Small Colony Variants of Pseudomonas aeruginosa Display Heterogeneity in Inhibiting Aspergillus fumigatus Biofilm.Mycopathologia. 2018 Feb;183(1):263-272. doi: 10.1007/s11046-017-0186-9. Epub 2017 Aug 7. Mycopathologia. 2018. PMID: 28785939
-
Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation.FEMS Microbiol Lett. 2010 Dec;313(2):96-102. doi: 10.1111/j.1574-6968.2010.02130.x. Epub 2010 Oct 22. FEMS Microbiol Lett. 2010. PMID: 20964704
-
Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics.mBio. 2019 Jul 30;10(4):e01501-19. doi: 10.1128/mBio.01501-19. mBio. 2019. PMID: 31363032 Free PMC article.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2020 Jun 10;6(6):CD009528. doi: 10.1002/14651858.CD009528.pub5. Cochrane Database Syst Rev. 2020. PMID: 32520436 Free PMC article.
-
Aspergillus-Pseudomonas interaction, relevant to competition in airways.Med Mycol. 2019 Apr 1;57(Supplement_2):S228-S232. doi: 10.1093/mmy/myy087. Med Mycol. 2019. PMID: 30816973 Review.
Cited by
-
Analysis of the Aspergillus fumigatus Biofilm Extracellular Matrix by Solid-State Nuclear Magnetic Resonance Spectroscopy.Eukaryot Cell. 2015 Nov;14(11):1064-72. doi: 10.1128/EC.00050-15. Epub 2015 Jul 10. Eukaryot Cell. 2015. PMID: 26163318 Free PMC article.
-
Effect of acute predation with bacteriophage on intermicrobial aggression by Pseudomonas aeruginosa.PLoS One. 2017 Jun 16;12(6):e0179659. doi: 10.1371/journal.pone.0179659. eCollection 2017. PLoS One. 2017. PMID: 28622385 Free PMC article.
-
Fungal biofilm formation and its regulatory mechanism.Heliyon. 2024 Jun 12;10(12):e32766. doi: 10.1016/j.heliyon.2024.e32766. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38988529 Free PMC article. Review.
-
Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis.PeerJ. 2018 Nov 9;6:e5931. doi: 10.7717/peerj.5931. eCollection 2018. PeerJ. 2018. PMID: 30430043 Free PMC article.
-
Effects of Propidium Monoazide (PMA) Treatment on Mycobiome and Bacteriome Analysis of Cystic Fibrosis Airways during Exacerbation.PLoS One. 2016 Dec 28;11(12):e0168860. doi: 10.1371/journal.pone.0168860. eCollection 2016. PLoS One. 2016. PMID: 28030619 Free PMC article.
References
-
- Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One. 2011;6:e28654. doi: 10.1371/journal.pone.0028654. - DOI - PMC - PubMed
-
- Duggal R, Rajwanshi A, Gupta N, Lal A, Singhal M. Polymicrobial lung infection in postrenal transplant recipient diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2010;38:294–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

