Senescence-secreted factors activate Myc and sensitize pretransformed cells to TRAIL-induced apoptosis
- PMID: 24589226
- PMCID: PMC4326894
- DOI: 10.1111/acel.12197
Senescence-secreted factors activate Myc and sensitize pretransformed cells to TRAIL-induced apoptosis
Abstract
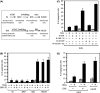
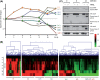
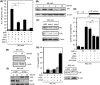
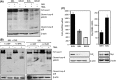
Senescent cells secrete a plethora of factors with potent paracrine signaling capacity. Strikingly, senescence, which acts as defense against cell transformation, exerts pro-tumorigenic activities through its secretome by promoting tumor-specific features, such as cellular proliferation, epithelial-mesenchymal transition and invasiveness. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has the unique activity of activating cell death exclusively in tumor cells. Given that the senescence-associated secretome (SAS) supports cell transformation, we asked whether SAS factor(s) would establish a program required for the acquisition of TRAIL sensitivity. We found that conditioned media from several types of senescent cells (CMS) efficiently sensitized pretransformed cells to TRAIL, while the same was not observed with normal or immortalized cells. Dynamic transcription profiling of CMS-exposed pretransformed cells indicated a paracrine autoregulatory loop of SAS factors and a dominant role of CMS-induced MYC. Sensitization to TRAIL coincided with and depended on MYC upregulation and massive changes in gene regulation. Senescent cell-induced MYC silenced its target gene CFLAR, encoding the apoptosis inhibitor FLIPL , thus leading to the acquisition of TRAIL sensitivity. Altogether, our results reveal that senescent cell-secreted factors exert a TRAIL-sensitizing effect on pretransformed cells by modulating the expression of MYC and CFLAR. Notably, CMS dose-dependent sensitization to TRAIL was observed with TRAIL-insensitive cancer cells and confirmed in co-culture experiments. Dissection and characterization of TRAIL-sensitizing CMS factors and the associated signaling pathway(s) will not only provide a mechanistic insight into the acquisition of TRAIL sensitivity but may lead to novel concepts for apoptogenic therapies of premalignant and TRAIL-resistant tumors.
Keywords: Myc; TRAIL; apoptosis; secretome; senescence; tumor.
© 2014 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

References
-
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Ashkenazi AHP, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J. Clin. Oncol. 2008;26:3621–3630. - PubMed
-
- Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. - PubMed
-
- Campisi J. Aging and cancer: the double-edged sword of replicative senescence. J. Am. Geriatr. Soc. 1997;45:482–488. - PubMed
-
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

