Semaphorin 3A: A new player in bone remodeling
- PMID: 24589620
- PMCID: PMC3974794
- DOI: 10.4161/cam.27752
Semaphorin 3A: A new player in bone remodeling
Abstract
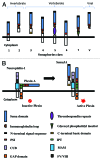
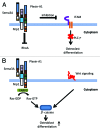
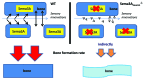
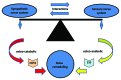
Semaphorin 3A (Sema3A) is a protein identified originally as a diffusible axonal chemorepellent. Sema3A has multifunctional roles in embryonic development, immune regulation, vascularization, and oncogenesis. Bone remodeling consists of two phases: the removal of mineralized bone by osteoclasts and the formation of new bone by osteoblasts, and plays an essential role in skeletal diseases such as osteoporosis. Recent studies have shown that Sema3A is implicated in the regulation of osteoblastgenesis and osteoclastgenesis. Moreover, low bone mass in mice with specific knockout of Sema3A in the neurons indicates that Sema3A regulates bone remodeling indirectly. This review highlights recent advances on our understanding of the role of sema3A as a new player in the regulation of bone remodeling and proposes the potential of sema3A in the diagnosis and therapy of bone diseases.
Keywords: Semaphorin 3A; bone remodeling; osteoblast; osteoclast; sensory innervation.
Figures
Similar articles
-
A Potential Role of Semaphorin 3A during Orthodontic Tooth Movement.Int J Mol Sci. 2021 Aug 2;22(15):8297. doi: 10.3390/ijms22158297. Int J Mol Sci. 2021. PMID: 34361063 Free PMC article.
-
Sema3A regulates bone-mass accrual through sensory innervations.Nature. 2013 May 23;497(7450):490-3. doi: 10.1038/nature12115. Epub 2013 May 5. Nature. 2013. PMID: 23644455
-
The Role Of Semaphorin 3A In The Skeletal System.Crit Rev Eukaryot Gene Expr. 2015;25(1):47-57. doi: 10.1615/critreveukaryotgeneexpr.2015012469. Crit Rev Eukaryot Gene Expr. 2015. PMID: 25955818 Review.
-
Adenosine A2A receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis.FASEB J. 2018 Jul;32(7):3487-3501. doi: 10.1096/fj.201700217R. Epub 2018 Feb 2. FASEB J. 2018. PMID: 29394106 Free PMC article.
-
Semaphorin 3A on Osteoporosis: An Overreview of the Literature.Calcif Tissue Int. 2025 Feb 22;116(1):43. doi: 10.1007/s00223-025-01350-4. Calcif Tissue Int. 2025. PMID: 39985619 Review.
Cited by
-
Formation and Developmental Specification of the Odontogenic and Osteogenic Mesenchymes.Front Cell Dev Biol. 2020 Jul 17;8:640. doi: 10.3389/fcell.2020.00640. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850793 Free PMC article. Review.
-
Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A.Bone. 2020 May;134:115260. doi: 10.1016/j.bone.2020.115260. Epub 2020 Feb 3. Bone. 2020. PMID: 32028017 Free PMC article.
-
Transcriptomic Signature and Pro-Osteoclastic Secreted Factors of Abnormal Bone-Marrow Stromal Cells in Fibrous Dysplasia.Cells. 2024 Apr 30;13(9):774. doi: 10.3390/cells13090774. Cells. 2024. PMID: 38727310 Free PMC article.
-
The Influence of Radiation on Bone and Bone Cells-Differential Effects on Osteoclasts and Osteoblasts.Int J Mol Sci. 2020 Sep 2;21(17):6377. doi: 10.3390/ijms21176377. Int J Mol Sci. 2020. PMID: 32887421 Free PMC article. Review.
-
Local Application of Semaphorin 3A Combined with Adipose-Derived Stem Cell Sheet and Anorganic Bovine Bone Granules Enhances Bone Regeneration in Type 2 Diabetes Mellitus Rats.Stem Cells Int. 2019 Jul 31;2019:2506463. doi: 10.1155/2019/2506463. eCollection 2019. Stem Cells Int. 2019. PMID: 31467560 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
