Identifying functional anti-Staphylococcus aureus antibodies by sequencing antibody repertoires of patient plasmablasts
- PMID: 24589749
- PMCID: PMC4066023
- DOI: 10.1016/j.clim.2014.02.010
Identifying functional anti-Staphylococcus aureus antibodies by sequencing antibody repertoires of patient plasmablasts
Abstract
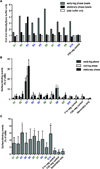
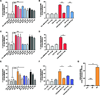
Infection by Staphylococcus aureus is on the rise, and there is a need for a better understanding of host immune responses that combat S. aureus. Here we use DNA barcoding to enable deep sequencing of the paired heavy- and light-chain immunoglobulin genes expressed by individual plasmablasts derived from S. aureus-infected humans. Bioinformatic analysis of the antibody repertoires revealed clonal families of heavy-chain sequences and enabled rational selection of antibodies for recombinant expression. Of the ten recombinant antibodies produced, seven bound to S. aureus, of which four promoted opsonophagocytosis of S. aureus. Five of the antibodies bound to known S. aureus cell-surface antigens, including fibronectin-binding protein A. Fibronectin-binding protein A-specific antibodies were isolated from two independent S. aureus-infected patients and mediated neutrophil killing of S. aureus in in vitro assays. Thus, our DNA barcoding approach enabled efficient identification of antibodies involved in protective host antibody responses against S. aureus.
Keywords: Antibody; Infection; Staphylococcus aureus.
Published by Elsevier Inc.
Figures




References
-
- Talan DA, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients 2004 and 2008. Clin Infect Dis. 2011;53(2):144–149. - PubMed
-
- Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. - PubMed
-
- Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54(8):1179–1186. - PubMed
-
- Holtfreter S, Kolata J, Broker BM. Towards the immune proteome of Staphylococcus aureus - The anti-S. aureus antibody response. Int J Med Microbiol. 2010;300(2–3):176–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

