Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice
- PMID: 24589889
- PMCID: PMC4311886
- DOI: 10.1038/mp.2014.10
Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice
Abstract
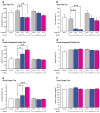
Pharmacologic blockade of monoamine oxidase A (MAOA) or serotonin transporter (5-HTT) has antidepressant and anxiolytic efficacy in adulthood. Yet, genetically conferred MAOA or 5-HTT hypoactivity is associated with altered aggression and increased anxiety/depression. Here we test the hypothesis that increased monoamine signaling during development causes these paradoxical aggressive and affective phenotypes. We find that pharmacologic MAOA blockade during early postnatal development (P2-P21) but not during peri-adolescence (P22-41) increases anxiety- and depression-like behavior in adult (>P90) mice, mimicking the effect of P2-21 5-HTT inhibition. Moreover, MAOA blockade during peri-adolescence, but not P2-21 or P182-201, increases adult aggressive behavior, and 5-HTT blockade from P22-P41 reduced adult aggression. Blockade of the dopamine transporter, but not the norepinephrine transporter, during P22-41 also increases adult aggressive behavior. Thus, P2-21 is a sensitive period during which 5-HT modulates adult anxiety/depression-like behavior, and P22-41 is a sensitive period during which DA and 5-HT bi-directionally modulate adult aggression. Permanently altered DAergic function as a consequence of increased P22-P41 monoamine signaling might underlie altered aggression. In support of this hypothesis, we find altered aggression correlating positively with locomotor response to amphetamine challenge in adulthood. Proving that altered DA function and aggression are causally linked, we demonstrate that optogenetic activation of VTA DAergic neurons increases aggression. It therefore appears that genetic and pharmacologic factors impacting dopamine and serotonin signaling during sensitive developmental periods can modulate adult monoaminergic function and thereby alter risk for aggressive and emotional dysfunction.
Conflict of interest statement
Figures
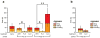

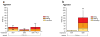


References
-
- Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol. 2007;7(1):8–17. - PubMed
-
- Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33(1):134–140. - PubMed
-
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. - PubMed
-
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

