Efficacy of intermittent combined RAF and MEK inhibition in a patient with concurrent BRAF- and NRAS-mutant malignancies
- PMID: 24589925
- PMCID: PMC4850735
- DOI: 10.1158/2159-8290.CD-13-1038
Efficacy of intermittent combined RAF and MEK inhibition in a patient with concurrent BRAF- and NRAS-mutant malignancies
Abstract
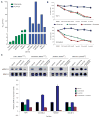
Vemurafenib, a RAF inhibitor, extends survival in patients with BRAF(V600)-mutant melanoma but activates extracellular signal-regulated kinase (ERK) signaling in RAS-mutant cells. In a patient with a BRAF(V600K)-mutant melanoma responding to vemurafenib, we observed accelerated progression of a previously unrecognized NRAS-mutant leukemia. We hypothesized that combining vemurafenib with a MAP-ERK kinase (MEK) inhibitor would inhibit ERK activation in the melanoma and prevent ERK activation by vemurafenib in the leukemia, and thus suppress both malignancies. We demonstrate that intermittent administration of vemurafenib led to a near-complete remission of the melanoma, and the addition of the MEK inhibitor cobimetinib (GDC-0973) caused suppression of vemurafenib-induced leukemic proliferation and ERK activation. Antimelanoma and antileukemia responses have been maintained for nearly 20 months, as documented by serial measurements of tumor-derived DNA in plasma in addition to conventional radiographic and clinical assessments of response. These data support testing of intermittent ERK pathway inhibition in the therapy for both RAS-mutant leukemia and BRAF-mutant melanoma.
Conflict of interest statement
Margaret K. Callahan has received a commercial research grant from Bristol-Myers Squibb and is a consultant/advisory board member of GlaxoSmithKline and Bristol-Myers Squibb. N. Rosen has received a commercial research grant from Chugai and is a consultant/advisory board member of Chugai and AstraZeneca. P.B. Chapman is a consultant/advisory board member of Genentech. No potential conflicts of interest were disclosed by the other authors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

