Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation
- PMID: 24590071
- PMCID: PMC4111109
- DOI: 10.1038/nature13070
Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation
Abstract
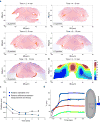
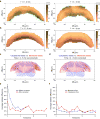
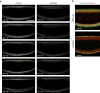
Epithelial folding mediated by apical constriction converts flat epithelial sheets into multilayered, complex tissue structures and is used throughout development in most animals. Little is known, however, about how forces produced near the apical surface of the tissue are transmitted within individual cells to generate the global changes in cell shape that characterize tissue deformation. Here we apply particle tracking velocimetry in gastrulating Drosophila embryos to measure the movement of cytoplasm and plasma membrane during ventral furrow formation. We find that cytoplasmic redistribution during the lengthening phase of ventral furrow formation can be precisely described by viscous flows that quantitatively match the predictions of hydrodynamics. Cell membranes move with the ambient cytoplasm, with little resistance to, or driving force on, the flow. Strikingly, apical constriction produces similar flow patterns in mutant embryos that fail to form cells before gastrulation ('acellular' embryos), such that the global redistribution of cytoplasm mirrors the summed redistribution occurring in individual cells of wild-type embryos. Our results indicate that during the lengthening phase of ventral furrow formation, hydrodynamic behaviour of the cytoplasm provides the predominant mechanism transmitting apically generated forces deep into the tissue and that cell individualization is dispensable.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

