Emerging roles of lymphatic endothelium in regulating adaptive immunity
- PMID: 24590280
- PMCID: PMC3938271
- DOI: 10.1172/JCI73316
Emerging roles of lymphatic endothelium in regulating adaptive immunity
Abstract
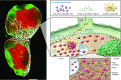
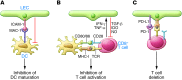
Emerging research on the roles of stromal cells in modulating adaptive immune responses has included a new focus on lymphatic endothelial cells (LECs). LECs are presumably the first cells that come into direct contact with peripheral antigens, cytokines, danger signals, and immune cells travelling from peripheral tissues to lymph nodes. LECs can modulate dendritic cell function, present antigens to T cells on MHC class I and MHC class II molecules, and express immunomodulatory cytokines and receptors, which suggests that their roles in adaptive immunity are far more extensive than previously realized. This Review summarizes the emergent evidence that LECs are important in maintaining peripheral tolerance, limiting and resolving effector T cell responses, and modulating leukocyte function.
Figures

References
-
- Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70(4):987–1028. - PubMed
-
- Randolph GJ, Angeli V, Swartz MA. Dendritic cell trafficking to lymph nodes via lymphatic vessels. Nat Rev Immunol. 2005;5(8):1–12. - PubMed
-
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176(6):3578–3584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

