Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis
- PMID: 24590287
- PMCID: PMC3973074
- DOI: 10.1172/JCI71195
Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis
Abstract
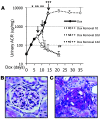
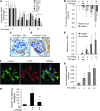
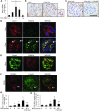
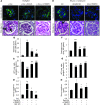
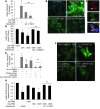
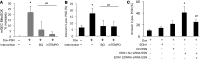
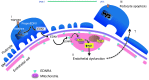
Focal segmental glomerular sclerosis (FSGS) is a primary kidney disease that is commonly associated with proteinuria and progressive loss of glomerular function, leading to development of chronic kidney disease (CKD). FSGS is characterized by podocyte injury and depletion and collapse of glomerular capillary segments. Progression of FSGS is associated with TGF-β activation in podocytes; however, it is not clear how TGF-β signaling promotes disease. Here, we determined that podocyte-specific activation of TGF-β signaling in transgenic mice and BALB/c mice with Adriamycin-induced glomerulosclerosis is associated with endothelin-1 (EDN1) release by podocytes, which mediates mitochondrial oxidative stress and dysfunction in adjacent endothelial cells via paracrine EDN1 receptor type A (EDNRA) activation. Endothelial dysfunction promoted podocyte apoptosis, and inhibition of EDNRA or scavenging of mitochondrial-targeted ROS prevented podocyte loss, albuminuria, glomerulosclerosis, and renal failure. We confirmed reciprocal crosstalk between podocytes and endothelial cells in a coculture system. Biopsies from patients with FSGS exhibited increased mitochondrial DNA damage, consistent with EDNRA-mediated glomerular endothelial mitochondrial oxidative stress. Our studies indicate that segmental glomerulosclerosis develops as a result of podocyte-endothelial crosstalk mediated by EDN1/EDNRA-dependent mitochondrial dysfunction and suggest that targeting the reciprocal interaction between podocytes and endothelia may provide opportunities for therapeutic intervention in FSGS.
Figures


References
-
- United States Renal Data System. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. USRDS 2011 Annual Data Report. In: NIH, ed. Bethesda, Maryland, USA: National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

