Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab
- PMID: 24590637
- PMCID: PMC4811023
- DOI: 10.1200/JCO.2013.53.0105
Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab
Abstract
Purpose: Programmed cell death 1 (PD-1) is an inhibitory receptor expressed by activated T cells that downmodulates effector functions and limits the generation of immune memory. PD-1 blockade can mediate tumor regression in a substantial proportion of patients with melanoma, but it is not known whether this is associated with extended survival or maintenance of response after treatment is discontinued.
Patients and methods: Patients with advanced melanoma (N = 107) enrolled between 2008 and 2012 received intravenous nivolumab in an outpatient setting every 2 weeks for up to 96 weeks and were observed for overall survival, long-term safety, and response duration after treatment discontinuation.
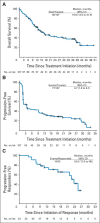
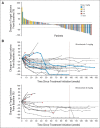
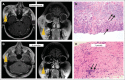
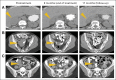
Results: Median overall survival in nivolumab-treated patients (62% with two to five prior systemic therapies) was 16.8 months, and 1- and 2-year survival rates were 62% and 43%, respectively. Among 33 patients with objective tumor regressions (31%), the Kaplan-Meier estimated median response duration was 2 years. Seventeen patients discontinued therapy for reasons other than disease progression, and 12 (71%) of 17 maintained responses off-therapy for at least 16 weeks (range, 16 to 56+ weeks). Objective response and toxicity rates were similar to those reported previously; in an extended analysis of all 306 patients treated on this trial (including those with other cancer types), exposure-adjusted toxicity rates were not cumulative.
Conclusion: Overall survival following nivolumab treatment in patients with advanced treatment-refractory melanoma compares favorably with that in literature studies of similar patient populations. Responses were durable and persisted after drug discontinuation. Long-term safety was acceptable. Ongoing randomized clinical trials will further assess the impact of nivolumab therapy on overall survival in patients with metastatic melanoma.
Trial registration: ClinicalTrials.gov NCT00730639.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Republished in
-
Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab.J Clin Oncol. 2023 Feb 10;41(5):943-954. doi: 10.1200/JCO.22.02272. J Clin Oncol. 2023. PMID: 36750016 Clinical Trial.
Comment in
-
Nivolumab: promising survival signal coupled with limited toxicity raises expectations.J Clin Oncol. 2014 Apr 1;32(10):986-8. doi: 10.1200/JCO.2013.54.5996. Epub 2014 Mar 3. J Clin Oncol. 2014. PMID: 24590655 Free PMC article. No abstract available.
-
Improved survival ends nivolumab trial early.Cancer Discov. 2014 Sep;4(9):979-80. doi: 10.1158/2159-8290.CD-NB2014-104. Epub 2014 Jul 9. Cancer Discov. 2014. PMID: 25185170
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

