Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio)
- PMID: 24591153
- PMCID: PMC4038790
- DOI: 10.1093/toxsci/kfu036
Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio)
Abstract
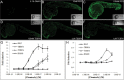
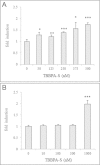
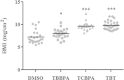
Obesity has increased dramatically over the past decades, reaching epidemic proportions. The reasons are likely multifactorial. One of the suggested causes is the accelerated exposure to obesity-inducing chemicals (obesogens). However, out of the tens of thousands of industrial chemicals humans are exposed to, very few have been tested for their obesogenic potential, mostly due to the limited availability of appropriate in vivo screening models. In this study, we investigated whether two commonly used flame retardants, the halogenated bisphenol-A (BPA) analogs tetrabromobisphenol-A (TBBPA) and tetrachlorobisphenol-A (TCBPA), could act as obesogens using zebrafish larvae as an in vivo animal model. The effect of embryonic exposure to these chemicals on lipid accumulation was analyzed by Oil Red-O staining, and correlated to their capacity to activate human and zebrafish peroxisome proliferator-activated receptor gamma (PPARγ) in zebrafish and in reporter cell lines. Then, the metabolic fate of TBBPA and TCBPA in zebrafish larvae was analyzed by high-performance liquid chromatography (HPLC) . TBBPA and TCBPA were readily taken up by the fish embryo and both compounds were biotransformed to sulfate-conjugated metabolites. Both halogenated-BPAs, as well as TBBPA-sulfate induced lipid accumulation in zebrafish larvae. TBBPA and TCBPA also induced late-onset weight gain in juvenile zebrafish. These effects correlated to their capacity to act as zebrafish PPARγ agonists. Screening of chemicals for inherent obesogenic capacities through the zebrafish lipid accumulation model could facilitate prioritizing chemicals for further investigations in rodents, and ultimately, help protect humans from exposure to environmental obesogens.
Keywords: PPARγ; TBBPA; TCBPA; lipid; obesity; zebrafish.
Figures



References
-
- Aballay L. R., Eynard A. R., Diaz Mdel P., Navarro A., Munoz S. E. Overweight and obesity: A review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr. Rev. 2013;71:168–179. - PubMed
-
- Baillie-Hamilton P. F. Chemical toxins: A hypothesis to explain the global obesity epidemic. J. Altern. Complement. Med. 2002;8:185–192. - PubMed
-
- Cariou R., Antignac J. P., Zalko D., Berrebi A., Cravedi J. P., Maume D., Marchand P., Monteau F., Riu A., Andre F., et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

