Patterns of structural dynamics in RACK1 protein retained throughout evolution: a hydrogen-deuterium exchange study of three orthologs
- PMID: 24591271
- PMCID: PMC4005715
- DOI: 10.1002/pro.2448
Patterns of structural dynamics in RACK1 protein retained throughout evolution: a hydrogen-deuterium exchange study of three orthologs
Abstract
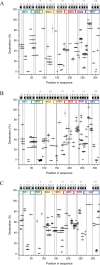
RACK1 is a member of the WD repeat family of proteins and is involved in multiple fundamental cellular processes. An intriguing feature of RACK1 is its ability to interact with at least 80 different protein partners. Thus, the structural features enabling such interactomic flexibility are of great interest. Several previous studies of the crystal structures of RACK1 orthologs described its detailed architecture and confirmed predictions that RACK1 adopts a seven-bladed β-propeller fold. However, this did not explain its ability to bind to multiple partners. We performed hydrogen-deuterium (H-D) exchange mass spectrometry on three orthologs of RACK1 (human, yeast, and plant) to obtain insights into the dynamic properties of RACK1 in solution. All three variants retained similar patterns of deuterium uptake, with some pronounced differences that can be attributed to RACK1's divergent biological functions. In all cases, the most rigid structural elements were confined to B-C turns and, to some extent, strands B and C, while the remaining regions retained much flexibility. We also compared the average rate constants for H-D exchange in different regions of RACK1 and found that amide protons in some regions exchanged at least 1000-fold faster than in others. We conclude that its evolutionarily retained structural architecture might have allowed RACK1 to accommodate multiple molecular partners. This was exemplified by our additional analysis of yeast RACK1 dimer, which showed stabilization, as well as destabilization, of several interface regions upon dimer formation.
Keywords: WD repeats; cell signaling; hydrogen deuterium exchange; mass spectrometry; protein dynamics; receptor for activated C kinase; scaffolding protein.
© 2014 The Protein Society.
Figures

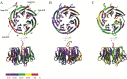


References
-
- Schloss JA. A Chlamydomonas gene encodes a G protein beta subunit-like polypeptide. Mol Gen Genet. 1990;221:443–452. - PubMed
-
- Kuo WN, Jones DL, Ku TW, Weeks KD, Jordon PM, Dopson NC. Immunoreactivity of PKC gammalambda and RACK1 in baker's yeast, lobster and wheat germ. Biochem Mol Biol Int. 1995;36:957–963. - PubMed
-
- Kwak JM, Kim SA, Lee SK, Oh SA, Byoun CH, Han JK, Nam HG. Insulin-induced maturation of Xenopus oocytes is inhibited by microinjection of a Brassica napus cDNA clone with high similarity to a mammalian receptor for activated protein kinase C. Planta. 1997;201:245–251. - PubMed
-
- Vani K, Yang G, Mohler J. Isolation and cloning of a Drosophila homolog to the mammalian RACK1 gene, implicated in PKC-mediated signalling. Biochim Biophys Acta. 1997;1358:67–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

