Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise
- PMID: 24591600
- PMCID: PMC3964070
- DOI: 10.1073/pnas.1400889111
Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise
Abstract
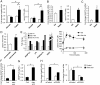
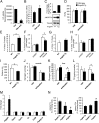
Physical activity increases energy metabolism in exercising muscle. Whether acute exercise elicits metabolic changes in nonexercising muscles remains unclear. We show that one of the few genes that is more highly induced in nonexercising muscle than in exercising human muscle during acute exercise encodes angiopoietin-like 4 (ANGPTL4), an inhibitor of lipoprotein lipase-mediated plasma triglyceride clearance. Using a combination of human, animal, and in vitro data, we show that induction of ANGPTL4 in nonexercising muscle is mediated by elevated plasma free fatty acids via peroxisome proliferator-activated receptor-δ, presumably leading to reduced local uptake of plasma triglyceride-derived fatty acids and their sparing for use by exercising muscle. In contrast, the induction of ANGPTL4 in exercising muscle likely is counteracted via AMP-activated protein kinase (AMPK)-mediated down-regulation, promoting the use of plasma triglycerides as fuel for active muscles. Our data suggest that nonexercising muscle and the local regulation of ANGPTL4 via AMPK and free fatty acids have key roles in governing lipid homeostasis during exercise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86(1):205–243. - PubMed
-
- Keller P, et al. Using systems biology to define the essential biological networks responsible for adaptation to endurance exercise training. Biochem Soc Trans. 2007;35(Pt 5):1306–1309. - PubMed
-
- Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107(4):463–471. - PubMed
-
- Schmutz S, et al. Endurance training modulates the muscular transcriptome response to acute exercise. Pflugers Arch. 2006;451(5):678–687. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

