Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients
- PMID: 24591638
- PMCID: PMC3964061
- DOI: 10.1073/pnas.1320753111
Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients
Abstract
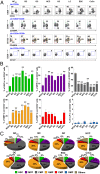
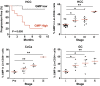
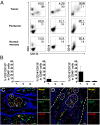
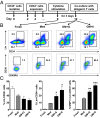
Cancer is associated with a profound perturbation in myelopoiesis that results in the accumulation of myeloid-derived suppressor cells (MDSCs) to promote disease progression. Recent studies in mice suggest that tumor-derived factors could regulate the differentiation of hematopoietic stem and progenitor cells (HSPCs) in the bone marrow and subsequently contribute to dysregulation of hematopoiesis. However, the nature and role of HPSCs in patients with cancer remain unknown. Here we show, in detailed studies of the peripheral blood from 133 untreated patients with seven different types of tumors, that the composition of circulating HSPCs was significantly altered in patients with solid tumors. The frequencies of circulating granulocyte-monocyte progenitors (GMPs) were increased four to seven fold in all types of tumors examined, and the circulating hematopoietic precursors exhibited myeloid bias with a skew toward granulocytic differentiation in patients with solid tumors. These myeloid precursors are selectively enriched in tumor tissues, and the high levels of circulating GMPs were positively correlated with disease progression. By using cord blood-derived CD34(+) cells, we developed an in vitro short-term culture model to effectively induce the rapid generation of MDSCs. We found that, among the factors produced by various tumors, GM-CSF, granulocyte colony-stimulating factor, and IL-6 could not only promote the myeloid-biased differentiation, but also induce the differentiation of myeloid precursors into functional MDSCs. These findings suggest that the altered circulating HSPCs may serve as an important link between dysregulated bone marrow hematopoiesis and accumulated MDSCs in patients with cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kondo M, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. - PubMed
-
- Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. - PubMed
-
- Essers MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–908. - PubMed
-
- Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

