PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India
- PMID: 24591775
- PMCID: PMC3928745
- DOI: 10.4103/0972-3919.125760
PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India
Abstract
Neuroendocrine tumors (NETs) are rare neoplasms characterized by overexpression of somatostatin receptors (SSTRs). Functional imaging plays a crucial role in management of NETs. Recently, positron emission tomography/computed tomography (PET/CT) with (68)Gallium ((68)Ga)-labeled somatostatin analogues has shown excellent results for imaging of NETs and better results than conventional SSTR scintigraphy. In this review we have discussed the utility of (68)Ga-labeled somatostatin analogue PET/CT in NETs for various established and potential indications. In addition we have also shared our own experience from a tertiary care center in India.
Keywords: 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-NaI3-octreotide; 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-Phe1-Tyr3-Octreotide; 68Gallium-labeled [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid]-Tyr3-Octreotate; Neuroendocrine tumor; PET/CT; somatostatin receptor.
Conflict of interest statement
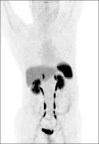
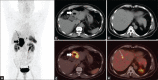
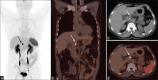
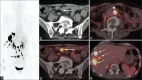
Figures
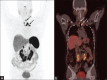
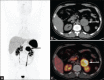
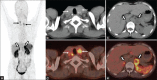
Similar articles
-
Role of Ga-68 DOTANOC Positron Emission Tomography/ Computed Tomography Scan in Clinical Management of Patients with Neuroendocrine Tumors and its Correlation with Conventional Imaging- Experience in a Tertiary Care Center in India.Indian J Nucl Med. 2022 Jan-Mar;37(1):29-36. doi: 10.4103/ijnm.ijnm_109_21. Epub 2022 Mar 25. Indian J Nucl Med. 2022. PMID: 35478677 Free PMC article.
-
Usefulness of Splenic Scintigraphy in Differentiating Splenosis and Malignancy on Gallium 68 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-NaI3-octreotide.World J Nucl Med. 2018 Jan-Mar;17(1):56-58. doi: 10.4103/wjnm.WJNM_1_17. World J Nucl Med. 2018. PMID: 29398968 Free PMC article.
-
PET and PET/CT with 68gallium-labeled somatostatin analogues in Non GEP-NETs Tumors.ScientificWorldJournal. 2014 Feb 13;2014:194123. doi: 10.1155/2014/194123. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24693229 Free PMC article.
-
68Ga-Labeled (1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetic acid)-1-NaI3-octreotide.2009 May 12 [updated 2009 Jun 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 May 12 [updated 2009 Jun 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641226 Free Books & Documents. Review.
-
Somatostatin Receptor Targeted PET-CT and Its Role in the Management and Theranostics of Gastroenteropancreatic Neuroendocrine Neoplasms.Diagnostics (Basel). 2023 Jun 24;13(13):2154. doi: 10.3390/diagnostics13132154. Diagnostics (Basel). 2023. PMID: 37443548 Free PMC article. Review.
Cited by
-
Role of Ga-68 DOTANOC Positron Emission Tomography/ Computed Tomography Scan in Clinical Management of Patients with Neuroendocrine Tumors and its Correlation with Conventional Imaging- Experience in a Tertiary Care Center in India.Indian J Nucl Med. 2022 Jan-Mar;37(1):29-36. doi: 10.4103/ijnm.ijnm_109_21. Epub 2022 Mar 25. Indian J Nucl Med. 2022. PMID: 35478677 Free PMC article.
-
Cushing Syndrome Secondary to Primary Neuroendocrine Lung Carcinoma.Case Rep Endocrinol. 2019 Jul 24;2019:1989260. doi: 10.1155/2019/1989260. eCollection 2019. Case Rep Endocrinol. 2019. PMID: 31428483 Free PMC article.
-
What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms?Korean J Radiol. 2019 Jan;20(1):5-17. doi: 10.3348/kjr.2018.0040. Epub 2018 Dec 27. Korean J Radiol. 2019. PMID: 30627018 Free PMC article. Review.
-
Case - Bladder paraganglioma in a pediatric patient.Can Urol Assoc J. 2018 May;12(5):E260-E264. doi: 10.5489/cuaj.4937. Epub 2018 Feb 6. Can Urol Assoc J. 2018. PMID: 29405904 Free PMC article. No abstract available.
-
A Comprehensive Review on Neuroendocrine Neoplasms: Presentation, Pathophysiology and Management.J Clin Med. 2023 Aug 5;12(15):5138. doi: 10.3390/jcm12155138. J Clin Med. 2023. PMID: 37568540 Free PMC article. Review.
References
-
- Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. - PubMed
-
- Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511. - PubMed
-
- Jensen RT. Endocrine tumors of the gastrointestinal tract and pancreas. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill; 2005. pp. 2220–31.
-
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

