Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease
- PMID: 24591834
- PMCID: PMC3937252
- DOI: 10.2147/NDT.S57909
Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease
Erratum in
- Neuropsychiatr Dis Treat. 2014;10:1997
Abstract
Background: Currently available treatments for Alzheimer's disease (AD) can produce mild improvements in cognitive function, behavior, and activities of daily living in patients, but their influence on long-term survival is not well established. This study was designed to assess patient survival and drug efficacy following a 2-year galantamine treatment in patients with mild to moderately severe AD.
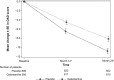
Methods: In this multicenter, double-blind study, patients were randomized 1:1 to receive galantamine or placebo. One primary end point was safety; mortality was assessed. An independent Data Safety Monitoring Board monitored mortality for the total deaths reaching prespecified numbers, using a time-to-event method and a Cox-regression model. The primary efficacy end point was cognitive change from baseline to month 24, as measured by the Mini-Mental State Examination (MMSE) score, analyzed using intent-to-treat analysis with the 'last observation carried forward' approach, in an analysis of covariance model.
Results: In all, 1,024 galantamine- and 1,021 placebo-treated patients received study drug, with mean age ~73 years, and mean (standard deviation [SD]) baseline MMSE score of 19 (4.08). A total of 32% of patients (661/2,045) completed the study, 27% (554/2,045) withdrew, and 41% (830/2,045) did not complete the study and were discontinued due to a Data Safety Monitoring Board-recommended early study termination. The mortality rate was significantly lower in the galantamine group versus placebo (hazard ratio [HR] =0.58; 95% confidence interval [CI]: 0.37; 0.89) (P=0.011). Cognitive impairment, based on the mean (SD) change in MMSE scores from baseline to month 24, significantly worsened in the placebo (-2.14 [4.34]) compared with the galantamine group (-1.41 [4.05]) (P<0.001). Functional impairment, based on mean (SD) change in the Disability Assessment in Dementia score (secondary end point), at month 24 significantly worsened in the placebo (-10.81 [18.27]) versus the galantamine group (-8.16 [17.25]) (P=0.002). Incidences of treatment-emergent adverse events were 54.0% for the galantamine and 48.6% for the placebo group.
Conclusion: Long-term treatment with galantamine significantly reduced mortality and the decline in cognition and daily living activities, in mild to moderate AD patients.
Identification: This study is registered at ClinicalTrials.gov (NCT00679627).
Keywords: cholinesterase inhibitors; cognition; long-term treatment; mortality; nicotinic.
Figures


References
-
- Alzheimer’s Association . 2013 Alzheimer’s Disease Facts and Figures. Washington, DC: Alzheimer’s Association; 2013. [Accessed March 25, 2013]. Available from: http://www.alz.org/alzheimers_disease_facts_and_figures.asp.
-
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158(9):1021–1031. - PubMed
-
- Feldman HH, Pirttila T, Dartigues JF, et al. Analyses of mortality risk in patients with dementia treated with galantamine. Acta Neurol Scand. 2009;119(1):22–31. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

