Cognitive-motor interactions of the basal ganglia in development
- PMID: 24592214
- PMCID: PMC3923298
- DOI: 10.3389/fnsys.2014.00016
Cognitive-motor interactions of the basal ganglia in development
Abstract
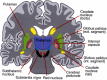
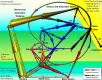
Neural circuits linking activity in anatomically segregated populations of neurons in subcortical structures and the neocortex throughout the human brain regulate complex behaviors such as walking, talking, language comprehension, and other cognitive functions associated with frontal lobes. The basal ganglia, which regulate motor control, are also crucial elements in the circuits that confer human reasoning and adaptive function. The basal ganglia are key elements in the control of reward-based learning, sequencing, discrete elements that constitute a complete motor act, and cognitive function. Imaging studies of intact human subjects and electrophysiologic and tracer studies of the brains and behavior of other species confirm these findings. We know that the relation between the basal ganglia and the cerebral cortical region allows for connections organized into discrete circuits. Rather than serving as a means for widespread cortical areas to gain access to the motor system, these loops reciprocally interconnect a large and diverse set of cerebral cortical areas with the basal ganglia. Neuronal activity within the basal ganglia associated with motor areas of the cerebral cortex is highly correlated with parameters of movement. Neuronal activity within the basal ganglia and cerebellar loops associated with the prefrontal cortex is related to the aspects of cognitive function. Thus, individual loops appear to be involved in distinct behavioral functions. Damage to the basal ganglia of circuits with motor areas of the cortex leads to motor symptoms, whereas damage to the subcortical components of circuits with non-motor areas of the cortex causes higher-order deficits. In this report, we review some of the anatomic, physiologic, and behavioral findings that have contributed to a reappraisal of function concerning the basal ganglia and cerebellar loops with the cerebral cortex and apply it in clinical applications to attention deficit/hyperactivity disorder (ADHD) with biomechanics and a discussion of retention of primitive reflexes being highly associated with the condition.
Keywords: ADHD; autism; basal ganglia; cognition; frontal lobe; posture.
Figures


References
-
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders. 4th Edn Washington, DC: American Psychiatric Press
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

