Polyamines in chemiosmosis in vivo: A cunning mechanism for the regulation of ATP synthesis during growth and stress
- PMID: 24592272
- PMCID: PMC3938100
- DOI: 10.3389/fpls.2014.00071
Polyamines in chemiosmosis in vivo: A cunning mechanism for the regulation of ATP synthesis during growth and stress
Abstract
Polyamines (PAs) are low molecular weight amines that occur in every living organism. The three main PAs (putrescine, spermidine, and spermine) are involved in several important biochemical processes covered in recent reviews. As rule of thumb, increase of the cellular titer of PAs in plants is related to cell growth and cell tolerance to abiotic and biotic stress. In the present contribution, we describe recent findings from plant bioenergetics that bring to light a previously unrecognized dynamic behavior of the PA pool. Traditionally, PAs are described by many authors as organic polycations, when in fact they are bases that can be found in a charged or uncharged form. Although uncharged forms represent less than 0.1% of the total pool, we propose that their physiological role could be crucial in chemiosmosis. This process describes the formation of a PA gradient across membranes within seconds and is difficult to be tested in vivo in plants due to the relatively small molecular weight of PAs and the speed of the process. We tested the hypothesis that PAs act as permeable buffers in intact leaves by using recent advances in vivo probing. We found that an increase of PAs increases the electric component (Δψ) and decreases the ΔpH component of the proton motive force. These findings reveal an important modulation of the energy production process and photoprotection of the chloroplast by PAs. We explain in detail the theory behind PA pumping and ion trapping in acidic compartments (such as the lumen in chloroplasts) and how this regulatory process could improve either the photochemical efficiency of the photosynthetic apparatus and increase the synthesis of ATP or fine tune antenna regulation and make the plant more tolerant to stress.
Keywords: ATP synthesis; chloroplast; photosynthesis; polyamines; proton motive force; putrescine; stress.
Figures
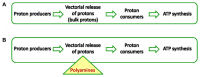
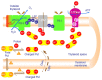

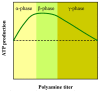
References
-
- Alcázar R., Planas J., Saxena T., Zarza X., Bortolotti C., Cuevas J., et al. (2010b). Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol. Biochem. 48 547–552 10.1016/j.plaphy.2010.02.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

