Recent developments in protein and peptide parenteral delivery approaches
- PMID: 24592957
- PMCID: PMC4130463
- DOI: 10.4155/tde.14.5
Recent developments in protein and peptide parenteral delivery approaches
Abstract
Discovery of insulin in the early 1900s initiated the research and development to improve the means of therapeutic protein delivery in patients. In the past decade, great emphasis has been placed on bringing protein and peptide therapeutics to market. Despite tremendous efforts, parenteral delivery still remains the major mode of administration for protein and peptide therapeutics. Other routes such as oral, nasal, pulmonary and buccal are considered more opportunistic rather than routine application. Improving biological half-life, stability and therapeutic efficacy is central to protein and peptide delivery. Several approaches have been tried in the past to improve protein and peptide in vitro/in vivo stability and performance. Approaches may be broadly categorized as chemical modification and colloidal delivery systems. In this review we have discussed various chemical approaches such as PEGylation, hyperglycosylation, mannosylation, and colloidal carriers including microparticles, nanoparticles, liposomes, carbon nanotubes and micelles for improving protein and peptide delivery. Recent developments on in situ thermosensitive gel-based protein and peptide delivery have also been described. This review summarizes recent developments on some currently existing approaches to improve stability, bioavailability and bioactivity of peptide and protein therapeutics following parenteral administration.
Figures
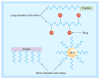


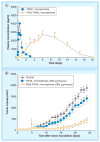
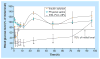
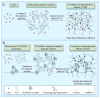

References
-
- Biologics research promises bolster future. www.phrma.org/media/releases/biologics-research-promises-bolster-future-....
-
- Schiffter HA. The Delivery of Drugs – Peptides and Proteins. In: Moo-Young M, editor. Comprehensive Biotechnology. Elsevier BV; Amsterdam, The Netherlands: 2011. pp. 587–604.
-
- Depreter F, Pilcer G, Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447(1–2):251–280. - PubMed
-
- Siekmeier R, Scheuch G. Inhaled insulin – does it become reality? J Physiol Pharmacol. 2008;59(Suppl. 6):81–113. - PubMed
-
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
