Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study
- PMID: 24593170
- PMCID: PMC4819587
- DOI: 10.3109/14397595.2014.881709
Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study
Abstract
Objectives: To evaluate the long-term efficacy and safety of certolizumab pegol (CZP) plus methotrexate treatment and to assess the efficacy of two CZP maintenance dosing schedules in Japanese rheumatoid arthritis (RA) patients with an inadequate response to methotrexate.
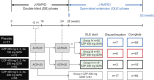
Methods: J-RAPID double-blind patients were entered into an open-label extension (OLE) study. Patients withdrawn due to lack of efficacy at 16 weeks and double-blind completers without a week-24 American College of Rheumatology (ACR) 20 response received CZP 200 mg every other week (Q2W) plus methotrexate. Double-blind completers with week-24 ACR20 responses were randomized to CZP 200 mg Q2W plus methotrexate or CZP 400 mg every 4 weeks plus methotrexate.
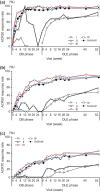
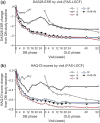
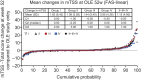
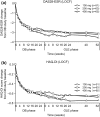
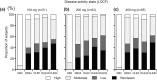
Results: The ACR20/ACR50/ACR70 response rates of double-blind completers (n = 204) were 89.7%/67.2%/36.3% at OLE entry and 95.6%/84.8%/58.3% at 52 weeks, respectively. Other clinical, functional and radiographic outcomes were sustained with long-term CZP plus methotrexate. Long-term treatment with CZP was well-tolerated with no new unexpected adverse events observed. The efficacy and safety of CZP treatment were similar between the two dosing schedules.
Conclusions: Continued CZP administration with methotrexate maintained efficacy over 52 weeks and was well-tolerated for Japanese RA patients. No obvious differences in clinical efficacy and safety were observed between the two dosing schedules, giving flexibility in maintenance administration schedules.
Keywords: Certolizumab pegol; Clinical study; Rheumatoid arthritis; TNF inhibitor; TNFα.
Figures

References
-
- Balanescu A, Wiland P. Maximizing early treatment with biologics in patients with rheumatoid arthritis: the ultimate breakthrough in joints preservation. Rheumatol Int. 2013;33((6)):1379–86. - PubMed
-
- Sharma P, Pathak K. Are biological targets the final goal for rheumatoid arthritis therapy? Expert Opin Biol Ther. 2012;12((12)):1611–22. - PubMed
-
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130((6)):478–86. - PubMed
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48((1)):35–45. - PubMed
-
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50((4)):1051–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

