Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies
- PMID: 24593228
- PMCID: PMC3997090
- DOI: 10.1089/hum.2013.214
Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies
Abstract
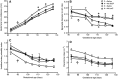
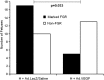
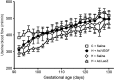
Fetal growth restriction (FGR) occurs in ∼8% of pregnancies and is a major cause of perinatal mortality and morbidity. There is no effective treatment. FGR is characterized by reduced uterine blood flow (UBF). In normal sheep pregnancies, local uterine artery (UtA) adenovirus (Ad)-mediated overexpression of vascular endothelial growth factor (VEGF) increases UBF. Herein we evaluated Ad.VEGF therapy in the overnourished adolescent ewe, an experimental paradigm in which reduced UBF from midgestation correlates with reduced lamb birthweight near term. Singleton pregnancies were established using embryo transfer in adolescent ewes subsequently offered a high intake (n=45) or control intake (n=12) of a complete diet to generate FGR or normal fetoplacental growth, respectively. High-intake ewes were randomized midgestation to receive bilateral UtA injections of 5×10¹¹ particles Ad.VEGF-A165 (n=18), control vector Ad.LacZ (n=14), or control saline (n=13). Fetal growth/well-being were evaluated using serial ultrasound. UBF was monitored using indwelling flowprobes until necropsy at 0.9 gestation. Vasorelaxation, neovascularization within the perivascular adventitia, and placental mRNA expression of angiogenic factors/receptors were examined using organ bath analysis, anti-vWF immunohistochemistry, and qRT-PCR, respectively. Ad.VEGF significantly increased ultrasonographic fetal growth velocity at 3-4 weeks postinjection (p=0.016-0.047). At 0.9 gestation fewer fetuses were markedly growth-restricted (birthweight >2SD below contemporaneous control-intake mean) after Ad.VEGF therapy. There was also evidence of mitigated fetal brain sparing (lower biparietal diameter-to-abdominal circumference and brain-to-liver weight ratios). No effects were observed on UBF or neovascularization; however, Ad.VEGF-transduced vessels demonstrated strikingly enhanced vasorelaxation. Placental efficiency (fetal-to-placental weight ratio) and FLT1/KDR mRNA expression were increased in the maternal but not fetal placental compartments, suggesting downstream effects on placental function. Ad.VEGF gene therapy improves fetal growth in a sheep model of FGR, although the precise mechanism of action remains unclear.
Figures

Similar articles
-
Local delivery of VEGF adenovirus to the uterine artery increases vasorelaxation and uterine blood flow in the pregnant sheep.Gene Ther. 2008 Oct;15(19):1344-50. doi: 10.1038/gt.2008.102. Epub 2008 Jun 19. Gene Ther. 2008. PMID: 18563186
-
Peri- and Postnatal Effects of Prenatal Adenoviral VEGF Gene Therapy in Growth-Restricted Sheep.Biol Reprod. 2016 Jun;94(6):142. doi: 10.1095/biolreprod.115.133744. Epub 2016 Apr 20. Biol Reprod. 2016. PMID: 27103444
-
Maternal Therapy with Ad.VEGF-A165 Increases Fetal Weight at Term in a Guinea-Pig Model of Fetal Growth Restriction.Hum Gene Ther. 2016 Dec;27(12):997-1007. doi: 10.1089/hum.2016.046. Epub 2016 Aug 16. Hum Gene Ther. 2016. PMID: 27530140
-
Maternal uterine artery VEGF gene therapy for treatment of intrauterine growth restriction.Placenta. 2017 Nov;59 Suppl 1:S44-S50. doi: 10.1016/j.placenta.2017.09.011. Epub 2017 Sep 27. Placenta. 2017. PMID: 29031540 Review.
-
Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus.Biol Reprod. 2004 Oct;71(4):1055-62. doi: 10.1095/biolreprod.104.030965. Epub 2004 Jun 16. Biol Reprod. 2004. PMID: 15201203 Review.
Cited by
-
The importance of nutrition in pregnancy and lactation: lifelong consequences.Am J Obstet Gynecol. 2022 May;226(5):607-632. doi: 10.1016/j.ajog.2021.12.035. Epub 2021 Dec 27. Am J Obstet Gynecol. 2022. PMID: 34968458 Free PMC article. Review.
-
EVERREST prospective study: a 6-year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction.BMC Pregnancy Childbirth. 2017 Jan 23;17(1):43. doi: 10.1186/s12884-017-1226-7. BMC Pregnancy Childbirth. 2017. PMID: 28114884 Free PMC article.
-
In Utero Gene Therapy (IUGT) Using GLOBE Lentiviral Vector Phenotypically Corrects the Heterozygous Humanised Mouse Model and Its Progress Can Be Monitored Using MRI Techniques.Sci Rep. 2019 Aug 12;9(1):11592. doi: 10.1038/s41598-019-48078-4. Sci Rep. 2019. PMID: 31406195 Free PMC article.
-
Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia.Nature. 2025 Jan;637(8045):412-421. doi: 10.1038/s41586-024-08291-2. Epub 2024 Dec 11. Nature. 2025. PMID: 39663452 Free PMC article.
-
From Pre-Clinical Studies to Clinical Trials: Generation of Novel Therapies for Pregnancy Complications.Int J Mol Sci. 2015 Jun 8;16(6):12907-24. doi: 10.3390/ijms160612907. Int J Mol Sci. 2015. PMID: 26062129 Free PMC article. Review.
References
-
- Barker D.J. (2006). Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 49, 270–283 - PubMed
-
- Bernstein I.M., et al. (2000). Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 182, 198–206 - PubMed
-
- Carr D.J., et al. (2011). Ultrasonographic assessment of growth and estimation of birthweight in late gestation fetal sheep. Ultrasound Med. Biol. 37, 1588–1595 - PubMed
-
- Carr D.J., et al. (2012). Fetoplacental biometry and umbilical artery Doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 207, 141.e6–141.e15 - PubMed
-
- Costeloe K., et al. (2000). The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 106, 659–671 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
