The effects of obesity, diabetes and metabolic syndrome on the hydrolytic enzymes of the endocannabinoid system in animal and human adipocytes
- PMID: 24593280
- PMCID: PMC3995979
- DOI: 10.1186/1476-511X-13-43
The effects of obesity, diabetes and metabolic syndrome on the hydrolytic enzymes of the endocannabinoid system in animal and human adipocytes
Abstract
Background: Circulating endocannabinoid levels are increased in obesity and diabetes. We have shown that fatty acid amide hydrolase (FAAH, an endocannabinoid hydrolysing enzyme) in subcutaneous adipose tissue positively correlates with BMI in healthy volunteers. The aim of the present study was to investigate whether the hydrolytic enzymes of the endocannabinoid system are affected by diabetes or metabolic syndrome in obesity.
Methods: Using radiolabelled substrates, FAAH and monoacylglycerol lipase (MGL) activities were assessed in adipocytes from various adipose depots in Zucker rats (n = 22, subcutaneous abdominal, visceral and epididymal) and bariatric patients (n = 28, subcutaneous abdominal and omental).
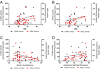
Results: FAAH activity was significantly increased in adipocytes of obese (Zucker Fatty) compared to Zucker lean rats (P < 0.05) but was not raised in the Zucker Diabetic Fatty rats (ZDF). MGL activity was raised in both Zucker Fatty (P < 0.001-0.01) and ZDF rats (P < 0.05) and was positively correlated with body weight and plasma glucose levels (P < 0.01). In bariatric patients (BMI range 37-58 kg.m²), there was a trend for MGL activity to correlate positively with BMI, reaching significance when type 2 diabetic patients were removed. FAAH and MGL activities in obese humans were not correlated with blood pressure, skinfold thicknesses, fasting glucose, insulin, HbA1c, triglycerides or cholesterol levels.
Conclusions: FAAH in adipocytes is differentially altered in animal models of obesity and diabetes, while MGL activity is increased by both. However, in obese humans, FAAH or MGL activity in adipocytes is not affected by diabetes, dyslipidaemia or other markers of metabolic dysfunction. This suggests increased circulating levels of endocannabinoids are not a result of altered degradation in adipose tissue.
Figures

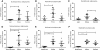



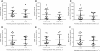
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

