Tobramycin exposure from active calcium sulfate bone graft substitute
- PMID: 24593819
- PMCID: PMC3944781
- DOI: 10.1186/2050-6511-15-12
Tobramycin exposure from active calcium sulfate bone graft substitute
Abstract
Background: Bone graft substitute such as calcium sulfate are frequently used as carrier material for local antimicrobial therapy in orthopedic surgery. This study aimed to assess the systemic absorption and disposition of tobramycin in patients treated with a tobramycin-laden bone graft substitute (Osteoset® T).
Methods: Nine blood samples were taken from 12 patients over 10 days after Osteoset® T surgical implantation. Tobramycin concentration was measured by fluorescence polarization. Population pharmacokinetic analysis was performed using NONMEM to assess the average value and variability (CV) of pharmacokinetic parameters. Bioavailability (F) was assessed by equating clearance (CL) with creatinine clearance (Cockcroft CLCr). Based on the final model, simulations with various doses and renal function levels were performed. (ClinicalTrials.gov number, NCT01938417).
Results: The patients were 52 +/- 20 years old, their mean body weight was 73 +/- 17 kg and their mean CLCr was 119 +/- 55 mL/min. Either 10 g or 20 g Osteoset® T with 4% tobramycin sulfate was implanted in various sites. Concentration profiles remained low and consistent with absorption rate-limited first-order release, while showing important variability. With CL equated to CLCr, mean absorption rate constant (ka) was 0.06 h-1, F was 63% or 32% (CV 74%) for 10 and 20 g Osteoset® T respectively, and volume of distribution (V) was 16.6 L (CV 89%). Simulations predicted sustained high, potentially toxic concentrations with 10 g, 30 g and 50 g Osteoset® T for CLCr values below 10, 20 and 30 mL/min, respectively.
Conclusions: Osteoset® T does not raise toxicity concerns in subjects without significant renal failure. The risk/benefit ratio might turn unfavorable in case of severe renal failure, even after standard dose implantation.
Figures
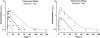
References
-
- Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The Pharmacokinetics of Simplex-tobramycin bone cement. J Bone Joint Surg. 2003;85-B:646–649. - PubMed
-
- Beal SL, Sheiner LB, Boeckmann AJ. NONMEM Users Guides 1989–2006. Ellicott City, Maryland, USA: Eds Icon Development Solutions;
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

