Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR
- PMID: 24594780
- PMCID: PMC4511388
- DOI: 10.1373/clinchem.2013.217240
Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR
Abstract
Background: Human herpesvirus 6 (HHV-6) latently infects a majority of adults. In about 1% of the population HHV-6 exists in a chromosomally integrated form (ciHHV-6) that resides in every somatic and germ cell and can be transmitted through the germ line. Patients with ciHHV-6 have been misdiagnosed and unnecessarily treated for active HHV-6 infection, sometimes with important side effects, based on results from quantitative molecular HHV-6 tests.
Methods: A droplet digital PCR (ddPCR) assay was developed to identify ciHHV-6 in cellular patient samples by precisely determining the ratio of HHV-6 to cellular DNA. We validated the assay on confirmed ciHHV-6 patient samples and a cell line derived from a ciHHV-6 patient, and we analyzed hematopoietic stem cell transplant patients suspected of having ciHHV-6. We additionally evaluated whether the assay could be applied to stored plasma samples from a study of clinical correlates of HHV-6.
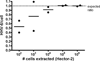
Results: The ddPCR assay accurately identified ciHHV-6 in cellular samples (buffy coat, peripheral blood mononuclear cells), giving a ratio very close to 1 HHV-6/cell [mean (SD), 1.02 (0.03)] in fluorescence in situ hybridization-confirmed sample). In stored plasma samples, the assay performance was set by design to have 100% sensitivity, which resulted in 82% specificity for ciHHV-6.
Conclusions: The possibility of ciHHV-6 is often overlooked in patients with detectable HHV-6 viral loads by quantitative PCR. Our ddPCR test provides rapid and accurate laboratory identification of ciHHV-6 from easily obtained cellular samples. In addition, the assay provides excellent sensitivity and specificity using stored plasma samples, facilitating retrospective analysis of the clinical significance of ciHHV-6.
Figures



References
-
- Zerr DM. Human herpesvirus 6 (hhv-6) disease in the setting of transplantation. Curr Opin Infect Dis. 2012;25:438–444. - PubMed
-
- Ward KN. The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J Clin Virol. 2005;32:183–193. - PubMed
-
- Leong HN, Tuke PW, Tedder RS, Khanom AB, Eglin RP, Atkinson CE, et al. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of uk blood donors. J Med Virol. 2007;79:45–51. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

