The chaperone BAG6 captures dislocated glycoproteins in the cytosol
- PMID: 24594942
- PMCID: PMC3940849
- DOI: 10.1371/journal.pone.0090204
The chaperone BAG6 captures dislocated glycoproteins in the cytosol
Abstract
Secretory and membrane (glyco)proteins are subject to quality control in the endoplasmic reticulum (ER) to ensure that only functional proteins reach their destination. Proteins deemed terminally misfolded and hence functionally defective may be dislocated to the cytosol, where the proteasome degrades them. What we know about this process stems mostly from overexpression of tagged misfolded proteins, or from situations where viruses have hijacked the quality control machinery to their advantage. We know of only very few endogenous substrates of ER quality control, most of which are degraded as part of a signaling pathway, such as Insig-1, but such examples do not necessarily represent terminally misfolded proteins. Here we show that endogenous dislocation clients are captured specifically in association with the cytosolic chaperone BAG6, or retrieved en masse via their glycan handle.
Conflict of interest statement
Figures

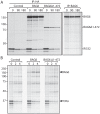
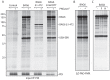

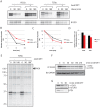
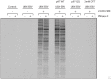
References
-
- Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

