Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching
- PMID: 24594970
- PMCID: PMC4108301
- DOI: 10.1038/ncomms4384
Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching
Erratum in
-
Author Correction: Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching.Nat Commun. 2019 Apr 18;10(1):1867. doi: 10.1038/s41467-019-09887-3. Nat Commun. 2019. PMID: 31000704 Free PMC article.
Abstract
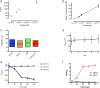
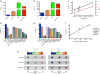
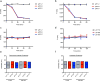
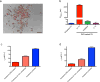
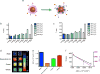
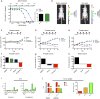
The effective delivery of therapeutics to disease sites significantly contributes to drug efficacy, toxicity and clearance. Here we demonstrate that clinically approved iron oxide nanoparticles (Ferumoxytol) can be utilized to carry one or multiple drugs. These so called 'nanophores' retain their cargo within their polymeric coating through weak electrostatic interactions and release it in slightly acidic conditions (pH 6.8 and below). The loading of drugs increases the nanophores' transverse T2 and longitudinal T1 nuclear magnetic resonance (NMR) proton relaxation times, which is proportional to amount of carried cargo. Chemotherapy with translational nanophores is more effective than the free drug in vitro and in vivo, without subjecting the drugs or the carrier nanoparticle to any chemical modification. Evaluation of cargo incorporation and payload levels in vitro and in vivo can be assessed via benchtop magnetic relaxometers, common NMR instruments or magnetic resonance imaging scanners.
Figures

References
-
- Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. - PubMed
-
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. - PubMed
-
- Huang PS, Oliff A. Drug-targeting strategies in cancer therapy. Curr Opin Genet Dev. 2001;11:104–110. - PubMed
-
- Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4:337–341. - PubMed
-
- Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

