Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic
- PMID: 24594998
- PMCID: PMC3974072
- DOI: 10.1038/bjc.2014.109
Uptake of tamoxifen in consecutive premenopausal women under surveillance in a high-risk breast cancer clinic
Abstract
Background: Randomised trials of tamoxifen versus placebo indicate that tamoxifen reduces breast cancer risk by approximately 33%, yet uptake is low. Approximately 10% of women in our clinic entered the IBIS-I prevention trial. We assess the uptake of tamoxifen in a consecutive series of premenopausal women not in a trial and explore the reasons for uptake through interviews.
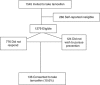
Methods: All eligible women between 33 and 46 years at ≥17% lifetime risk of breast cancer and undergoing annual mammography in our service were invited to take a 5-year course of tamoxifen. Reasons for accepting (n=15) or declining (n=15) were explored using semi-structured interviews.
Results: Of 1279 eligible women, 136 (10.6%) decided to take tamoxifen. Women >40 years (74 out of 553 (13.4%)) and those at higher non-BRCA-associated risk were more likely to accept tamoxifen (129 out of 1109 (11.6%)). Interviews highlighted four themes surrounding decision making: perceived impact of side effects, the impact of others' experience on beliefs about tamoxifen, tamoxifen as a 'cancer drug', and daily reminder of cancer risk.
Conclusions: Tamoxifen uptake was similar to previously ascertained uptake in a randomised controlled trial (IBIS-I). Concerns were similar in women who did or did not accept tamoxifen. Decision making appeared to be embedded in the experience of significant others.
Figures
References
-
- Altschuler A, Somkin CP. Women's decision making about whether or not to use breast cancer chemoprevention. Women Health. 2005;41:81–95. - PubMed
-
- Bastian LA, Lipkus AM, Kuchibhata MN, Weng HH, Halabi S, Ryan PD, Skinner CS, Rimer BK. Women's interest in chemoprevention for breast cancer. Arch Intern Med. 2001;161:1639–1644. - PubMed
-
- Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–4957. - PubMed
-
- Cheng WF, Lin HH, Torng PL, Huang SC. Comparison of endometrial changes among symptomatic tamoxifen-treated and non treated premenopausal and postmenopausal breast cancer patients. Gynecol Oncol. 1997;66:233–237. - PubMed
-
- Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A, International Breast Cancer Intervention Study I Investigators Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical