Arachidonic acid induction of Rho-mediated transendothelial migration in prostate cancer
- PMID: 24595005
- PMCID: PMC3992515
- DOI: 10.1038/bjc.2014.99
Arachidonic acid induction of Rho-mediated transendothelial migration in prostate cancer
Abstract
Background: Bone metastases in prostate cancer (CaP) result in CaP-related morbidity/mortality. The omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid (AA) and lipophilic statins affect metastasis-like behaviour in CaP cells, regulating the critical metastatic step of CaP migration to the bone marrow stroma.
Methods: Microscopic analysis and measurement of adhesion and invasion of CaP cells through bone marrow endothelial cells (BMEC) was undertaken with AA stimulation and/or simvastatin (SIM) treatment. Amoeboid characteristics of PC-3, PC3-GFP and DU-145 were analysed by western blotting and Rho assays.
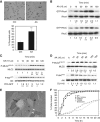
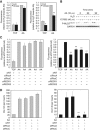
Results: The CaP cell lines PC-3, PC3-GFP and DU-145 share the ability to migrate across a BMEC layer. Specific amoeboid inhibition decreased transendothelial migration (TEM). AA stimulates amoeboid characteristics, driven by Rho signalling. Selective knock-down of components of the Rho pathway (RhoA, RhoC, Rho-associated protein kinase 1 (ROCK1) and ROCK2) showed that Rho signalling is crucial to TEM. Functions of these components were analysed, regarding adhesion to BMEC, migration in 2D and the induction of the amoeboid phenotype by AA. TEM was reduced by SIM treatment of PC3-GFP and DU-145, which inhibited Rho pathway signalling.
Conclusions: AA-induced TEM is mediated by the induction of a Rho-driven amoeboid phenotype. Inhibition of this cell migratory process may be an important therapeutic target in high-risk CaP.
Figures



Similar articles
-
Ligand-independent activation of EphA2 by arachidonic acid induces metastasis-like behaviour in prostate cancer cells.Br J Cancer. 2012 Nov 6;107(10):1737-44. doi: 10.1038/bjc.2012.457. Epub 2012 Oct 4. Br J Cancer. 2012. PMID: 23037715 Free PMC article.
-
Zoledronic Acid Inhibits the RhoA-mediated Amoeboid Motility of Prostate Cancer Cells.Curr Cancer Drug Targets. 2019;19(10):807-816. doi: 10.2174/1568009619666190115142858. Curr Cancer Drug Targets. 2019. PMID: 30648509
-
RhoA and RhoC have distinct roles in migration and invasion by acting through different targets.J Cell Biol. 2011 May 16;193(4):655-65. doi: 10.1083/jcb.201011038. J Cell Biol. 2011. PMID: 21576392 Free PMC article.
-
Functions of Rho family of small GTPases and Rho-associated coiled-coil kinases in bone cells during differentiation and mineralization.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):1009-1023. doi: 10.1016/j.bbagen.2017.02.005. Epub 2017 Feb 8. Biochim Biophys Acta Gen Subj. 2017. PMID: 28188861 Review.
-
RhoA/Rho-Kinase in the Cardiovascular System.Circ Res. 2016 Jan 22;118(2):352-66. doi: 10.1161/CIRCRESAHA.115.306532. Circ Res. 2016. PMID: 26838319 Review.
Cited by
-
Exosome-Derived miRNAs as Potential Biomarkers for Prostate Bone Metastasis.Int J Gen Med. 2022 Jun 1;15:5369-5383. doi: 10.2147/IJGM.S361981. eCollection 2022. Int J Gen Med. 2022. PMID: 35673634 Free PMC article.
-
KLF2 is a clinical diagnostic and treatment biomarker of breast cancer.Front Cell Dev Biol. 2023 Apr 13;11:1182123. doi: 10.3389/fcell.2023.1182123. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37123417 Free PMC article.
-
LEM domain containing 1 promotes oral squamous cell carcinoma invasion and endothelial transmigration.Br J Cancer. 2016 Jun 28;115(1):52-8. doi: 10.1038/bjc.2016.167. Epub 2016 Jun 9. Br J Cancer. 2016. PMID: 27280633 Free PMC article.
-
RhoA inhibits apoptosis and increases proliferation of cultured SPCA1 lung cancer cells.Mol Med Rep. 2017 Jun;15(6):3963-3968. doi: 10.3892/mmr.2017.6545. Epub 2017 May 3. Mol Med Rep. 2017. PMID: 28487954 Free PMC article.
-
Fatty Acid-Binding Protein 4-Mediated Regulation Is Pivotally Involved in Retinal Pathophysiology: A Review.Int J Mol Sci. 2024 Jul 14;25(14):7717. doi: 10.3390/ijms25147717. Int J Mol Sci. 2024. PMID: 39062961 Free PMC article. Review.
References
-
- Aktories K, Just I. Clostridial Rho-inhibiting protein toxins. Curr Top Microbiol Immunol. 2005;291:113–1145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

