KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability
- PMID: 24595108
- PMCID: PMC4063528
- DOI: 10.1126/scisignal.2005025
KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability
Abstract
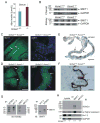
Na(+)-coupled solute transport is crucial for the uptake of nutrients and metabolic precursors, such as myo-inositol, an important osmolyte and precursor for various cell signaling molecules. We found that various solute transporters and potassium channel subunits formed complexes and reciprocally regulated each other in vitro and in vivo. Global metabolite profiling revealed that mice lacking KCNE2, a K(+) channel β subunit, showed a reduction in myo-inositol concentration in cerebrospinal fluid (CSF) but not in serum. Increased behavioral responsiveness to stress and seizure susceptibility in Kcne2(-/-) mice were alleviated by injections of myo-inositol. Suspecting a defect in myo-inositol transport, we found that KCNE2 and KCNQ1, a voltage-gated potassium channel α subunit, colocalized and coimmunoprecipitated with SMIT1, a Na(+)-coupled myo-inositol transporter, in the choroid plexus epithelium. Heterologous coexpression demonstrated that myo-inositol transport by SMIT1 was augmented by coexpression of KCNQ1 but was inhibited by coexpression of both KCNQ1 and KCNE2, which form a constitutively active, heteromeric K(+) channel. SMIT1 and the related transporter SMIT2 were also inhibited by a constitutively active mutant form of KCNQ1. The activities of KCNQ1 and KCNQ1-KCNE2 were augmented by SMIT1 and the glucose transporter SGLT1 but were suppressed by SMIT2. Channel-transporter signaling complexes may be a widespread mechanism to facilitate solute transport and electrochemical crosstalk.
Conflict of interest statement
Figures

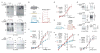
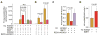

References
-
- McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. - PubMed
-
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–87. - PubMed
-
- Heron SE, Hernandez M, Edwards C, Edkins E, Jansen FE, Scheffer IE, Berkovic SF, Mulley JC. Neonatal seizures and long QT syndrome: a cardiocerebral channelopathy? Epilepsia. 2010;51:293–6. - PubMed
-
- Tam GW, van de Lagemaat LN, Redon R, Strathdee KE, Croning MD, Malloy MP, Muir WJ, Pickard BS, Deary IJ, Blackwood DH, Carter NP, Grant SG. Confirmed rare copy number variants implicate novel genes in schizophrenia. Biochem Soc Trans. 2010;38:445–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

