Expression of the effector protein IncD in Chlamydia trachomatis mediates recruitment of the lipid transfer protein CERT and the endoplasmic reticulum-resident protein VAPB to the inclusion membrane
- PMID: 24595143
- PMCID: PMC3993449
- DOI: 10.1128/IAI.01530-14
Expression of the effector protein IncD in Chlamydia trachomatis mediates recruitment of the lipid transfer protein CERT and the endoplasmic reticulum-resident protein VAPB to the inclusion membrane
Abstract
Chlamydia trachomatis is an obligate intracellular human pathogen responsible for ocular and genital infections. To establish its membrane-bound intracellular niche, the inclusion, C. trachomatis relies on a set of effector proteins that are injected into the host cells or inserted into the inclusion membrane. We previously proposed that insertion of the C. trachomatis effector protein IncD into the inclusion membrane contributes to the recruitment of the lipid transfer protein CERT to the inclusion. Due to the genetically intractable status of C. trachomatis at that time, this model of IncD-CERT interaction was inferred from ectopic expression of IncD and CERT in the host cell. In the present study, we investigated the impact of conditionally expressing a FLAG-tagged version of IncD in C. trachomatis. This genetic approach allowed us to establish that IncD-3×FLAG localized to the inclusion membrane and caused a massive recruitment of the lipid transfer protein CERT that relied on the PH domain of CERT. In addition, we showed that the massive IncD-dependent association of CERT with the inclusion led to an increased recruitment of the endoplasmic reticulum (ER)-resident protein VAPB, and we determined that, at the inclusion, CERT-VAPB interaction relied on the FFAT domain of CERT. Altogether, the data presented here show that expression of the C. trachomatis effector protein IncD mediates the recruitment of the lipid transfer protein CERT and the ER-resident protein VAPB to the inclusion.
Figures
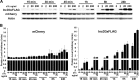


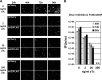
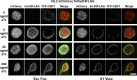
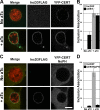
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

