Arginine 199 and leucine 208 have key roles in the control of adenosine A2A receptor signalling function
- PMID: 24595172
- PMCID: PMC3940607
- DOI: 10.1371/journal.pone.0089613
Arginine 199 and leucine 208 have key roles in the control of adenosine A2A receptor signalling function
Abstract
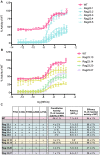
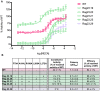
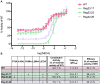
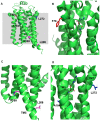
One successful approach to obtaining high-resolution crystal structures of G-protein coupled receptors is the introduction of thermostabilising mutations within the receptor. This technique allows the generation of receptor constructs stabilised into different conformations suitable for structural studies. Previously, we functionally characterised a number of mutants of the adenosine A2A receptor, thermostabilised either in an agonist or antagonist conformation, using a yeast cell growth assay and demonstrated that there is a correlation between thermostability and loss of constitutive activity. Here we report the functional characterisation of 30 mutants intermediate between the Rag23 (agonist conformation mutant) and the wild-type receptor using the same yeast signalling assay with the aim of gaining greater insight into the role individual amino acids have in receptor function. The data showed that R199 and L208 have important roles in receptor function; substituting either of these residues for alanine abolishes constitutive activity. In addition, the R199A mutation markedly reduces receptor potency while L208A reduces receptor efficacy. A184L and L272A mutations also reduce constitutive activity and potency although to a lesser extent than the R199A and L208A. In contrast, the F79A mutation increases constitutive activity, potency and efficacy of the receptor. These findings shed new light on the role individual residues have on stability of the receptor and also provide some clues as to the regions of the protein responsible for constitutive activity. Furthermore, the available adenosine A2A receptor structures have allowed us to put our findings into a structural context.
Conflict of interest statement
Figures


Similar articles
-
Loss of constitutive activity is correlated with increased thermostability of the human adenosine A2A receptor.Br J Pharmacol. 2013 Jul;169(5):988-98. doi: 10.1111/bph.12165. Br J Pharmacol. 2013. PMID: 23489072 Free PMC article.
-
Thermostabilisation of an agonist-bound conformation of the human adenosine A(2A) receptor.J Mol Biol. 2011 Jun 10;409(3):298-310. doi: 10.1016/j.jmb.2011.03.075. Epub 2011 Apr 9. J Mol Biol. 2011. PMID: 21501622 Free PMC article.
-
Dynamic behavior of the active and inactive states of the adenosine A(2A) receptor.J Phys Chem B. 2014 Mar 27;118(12):3355-65. doi: 10.1021/jp411618h. Epub 2014 Mar 17. J Phys Chem B. 2014. PMID: 24579769 Free PMC article.
-
Adenosine A2A receptor as a drug discovery target.J Med Chem. 2014 May 8;57(9):3623-50. doi: 10.1021/jm4011669. Epub 2013 Nov 15. J Med Chem. 2014. PMID: 24164628 Review.
-
Aspects of the general biology of adenosine A2A signaling.Prog Neurobiol. 2007 Dec;83(5):263-76. doi: 10.1016/j.pneurobio.2007.07.005. Epub 2007 Aug 1. Prog Neurobiol. 2007. PMID: 17804147 Review.
Cited by
-
Molecular Evidence of Adenosine Deaminase Linking Adenosine A2A Receptor and CD26 Proteins.Front Pharmacol. 2018 Feb 15;9:106. doi: 10.3389/fphar.2018.00106. eCollection 2018. Front Pharmacol. 2018. PMID: 29497379 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

