Brain pyroglutamate amyloid-β is produced by cathepsin B and is reduced by the cysteine protease inhibitor E64d, representing a potential Alzheimer's disease therapeutic
- PMID: 24595198
- PMCID: PMC4059604
- DOI: 10.3233/JAD-131370
Brain pyroglutamate amyloid-β is produced by cathepsin B and is reduced by the cysteine protease inhibitor E64d, representing a potential Alzheimer's disease therapeutic
Abstract
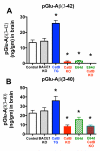
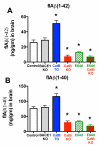
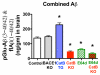
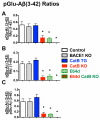
Pyroglutamate amyloid-β peptides (pGlu-Aβ) are particularly pernicious forms of amyloid-β peptides (Aβ) present in Alzheimer's disease (AD) brains. pGlu-Aβ peptides are N-terminally truncated forms of full-length Aβ peptides (flAβ(1-40/42)) in which the N-terminal glutamate is cyclized to pyroglutamate to generate pGlu-Aβ(3-40/42). β-secretase cleavage of amyloid-β precursor protein (AβPP) produces flAβ(1-40/42), but it is not yet known whether the β-secretase BACE1 or the alternative β-secretase cathepsin B (CatB) participate in the production of pGlu-Aβ. Therefore, this study examined the effects of gene knockout of these proteases on brain pGlu-Aβ levels in transgenic AβPPLon mice, which express AβPP isoform 695 and have the wild-type (wt) β-secretase activity found in most AD patients. Knockout or overexpression of the CatB gene reduced or increased, respectively, pGlu-Aβ(3-40/42), flAβ(1-40/42), and pGlu-Aβ plaque load, but knockout of the BACE1 gene had no effect on those parameters in the transgenic mice. Treatment of AβPPLon mice with E64d, a cysteine protease inhibitor of CatB, also reduced brain pGlu-Aβ(3-42), flAβ(1-40/42), and pGlu-Aβ plaque load. Treatment of neuronal-like chromaffin cells with CA074Me, an inhibitor of CatB, resulted in reduced levels of pGlu-Aβ(3-40) released from the activity-dependent, regulated secretory pathway. Moreover, CatB knockout and E64d treatment has been previously shown to improve memory deficits in the AβPPLon mice. These data illustrate the role of CatB in producing pGlu-Aβ and flAβ that participate as key factors in the development of AD. The advantages of CatB inhibitors, especially E64d and its derivatives, as alternatives to BACE1 inhibitors in treating AD patients are discussed.
Keywords: AβPP; BACE1; Pyroglutamate amyloid-β; cathepsin B; cysteine protease; inhibitor; protease; secretion; transgenic AD mice.
Figures




References
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Gandy S, Martins RN, Buxbaum J. Molecular and cellular basis for anti-amyloid therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:259–266. - PubMed
-
- De Kimpe L, Scheper W. From alpha to omega with Abeta: targeting the multiple molecular appearances of the pathogenic peptide in Alzheimer’s disease. Curr Med Chem. 2010;17:198–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

