Ccm3, a gene associated with cerebral cavernous malformations, is required for neuronal migration
- PMID: 24595293
- PMCID: PMC3943187
- DOI: 10.1242/dev.093526
Ccm3, a gene associated with cerebral cavernous malformations, is required for neuronal migration
Abstract
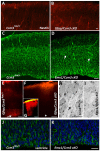
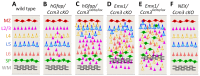
Loss of function of cerebral cavernous malformation 3 (CCM3) results in an autosomal dominant cerebrovascular disorder. Here, we uncover a developmental role for CCM3 in regulating neuronal migration in the neocortex. Using cell type-specific gene inactivation in mice, we show that CCM3 has both cell autonomous and cell non-autonomous functions in neural progenitors and is specifically required in radial glia and newly born pyramidal neurons migrating through the subventricular zone, but not in those migrating through the cortical plate. Loss of CCM3 function leads to RhoA activation, alterations in the actin and microtubule cytoskeleton affecting neuronal morphology, and abnormalities in laminar positioning of primarily late-born neurons, indicating CCM3 involvement in radial glia-dependent locomotion and possible interaction with the Cdk5/RhoA pathway. Thus, we identify a novel cytoplasmic regulator of neuronal migration and demonstrate that its inactivation in radial glia progenitors and nascent neurons produces severe malformations of cortical development.
Keywords: CCM3 (PDCD10); Cell autonomous function; Mouse; Nascent neurons; Neocortex; Radial glia.
Figures






References
-
- Alcamo E. A., Chirivella L., Dautzenberg M., Dobreva G., Fariñas I., Grosschedl R., McConnell S. K. (2008). Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377 - PubMed
-
- Angevine J. B., Jr, Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768 - PubMed
-
- Arlotta P., Molyneaux B. J., Chen J., Inoue J., Kominami R., Macklis J. D. (2005). Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45, 207–221 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

