Direct quantification of cell-free, circulating DNA from unpurified plasma
- PMID: 24595313
- PMCID: PMC3940427
- DOI: 10.1371/journal.pone.0087838
Direct quantification of cell-free, circulating DNA from unpurified plasma
Abstract
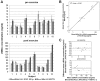
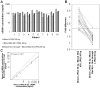
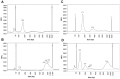
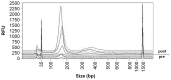
Cell-free DNA (cfDNA) in body tissues or fluids is extensively investigated in clinical medicine and other research fields. In this article we provide a direct quantitative real-time PCR (qPCR) as a sensitive tool for the measurement of cfDNA from plasma without previous DNA extraction, which is known to be accompanied by a reduction of DNA yield. The primer sets were designed to amplify a 90 and 222 bp multi-locus L1PA2 sequence. In the first module, cfDNA concentrations in unpurified plasma were compared to cfDNA concentrations in the eluate and the flow-through of the QIAamp DNA Blood Mini Kit and in the eluate of a phenol-chloroform isoamyl (PCI) based DNA extraction, to elucidate the DNA losses during extraction. The analyses revealed 2.79-fold higher cfDNA concentrations in unpurified plasma compared to the eluate of the QIAamp DNA Blood Mini Kit, while 36.7% of the total cfDNA were found in the flow-through. The PCI procedure only performed well on samples with high cfDNA concentrations, showing 87.4% of the concentrations measured in plasma. The DNA integrity strongly depended on the sample treatment. Further qualitative analyses indicated differing fractions of cfDNA fragment lengths in the eluate of both extraction methods. In the second module, cfDNA concentrations in the plasma of 74 coronary heart disease patients were compared to cfDNA concentrations of 74 healthy controls, using the direct L1PA2 qPCR for cfDNA quantification. The patient collective showed significantly higher cfDNA levels (mean (SD) 20.1 (23.8) ng/ml; range 5.1-183.0 ng/ml) compared to the healthy controls (9.7 (4.2) ng/ml; range 1.6-23.7 ng/ml). With our direct qPCR, we recommend a simple, economic and sensitive procedure for the quantification of cfDNA concentrations from plasma that might find broad applicability, if cfDNA became an established marker in the assessment of pathophysiological conditions.
Conflict of interest statement
Figures



References
-
- Van der Vaart M, Pretorius PJ (2008) Circulating DNA. Its Origin and Fluctuation. Ann N Y Acad Sci 1137: 18–26. - PubMed
-
- Schwarzenbach H, Hoon DS, Pantel K (2011) Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11 (6) 426–437. - PubMed
-
- Atamaniuk J, Vidotto C, Tschan H, Bachl N, Stuhlmeier KM, et al. (2004) Increased Concentrations of Cell-Free Plasma DNA after Exhaustive Exercise. Clin Chem 50 (9) 1668–1670. - PubMed
-
- Atamaniuk J, Stuhlmeier KM, Vidotto C, Tschan H, Dossenbach-Glaninger A, et al. (2008) Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. Eur J Appl Physiol 104 (4) 711–717. - PubMed
-
- Atamaniuk J, Vidotto C, Kinzlbauer M, Bachl N, Tiran B, et al. (2010) Cell-free plasma DNA and purine nucleotide degradation markers following weightlifting exercise. Eur J Physiol 110 (4) 695–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

