Brain barriers and a subpopulation of astroglial progenitors of developing human forebrain are immunostained for the glycoprotein YKL-40
- PMID: 24595665
- PMCID: PMC4005366
- DOI: 10.1369/0022155414528514
Brain barriers and a subpopulation of astroglial progenitors of developing human forebrain are immunostained for the glycoprotein YKL-40
Abstract
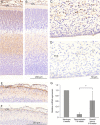
YKL-40, a glycoprotein involved in cell differentiation, has been associated with neurodevelopmental disorders, angiogenesis, neuroinflammation and glioblastomas. We evaluated YKL-40 protein distribution in the early human forebrain using double-labeling immunofluorescence and immunohistochemistry. Immunoreactivity was detected in neuroepithelial cells, radial glial end feet, leptomeningeal cells and choroid plexus epithelial cells. The subpial marginal zone was YKL-40-positive, particularly in the hippocampus, from an early beginning stage in its development. Blood vessels in the intermediate and subventricular zones showed specific YKL-40 reactivity confined to pericytes. Furthermore, a population of YKL-40-positive, small, rounded cells was identified in the ventricular and subventricular zones. Real-time quantitative RT-PCR analysis showed strong YKL-40 mRNA expression in the leptomeninges and the choroid plexuses, and weaker expression in the telencephalic wall. Immunohistochemistry revealed a differential distribution of YKL-40 across the zones of the developing telencephalic wall. We show that YKL-40 is associated with sites of the brain barrier systems and propose that it is involved in controlling local angiogenesis and access of peripheral cells to the forebrain via secretion from leptomeningeal cells, choroid plexus epithelium and pericytes. Furthermore, we suggest that the small, rounded, YKL-40-positive cells represent a subpopulation of astroglial progenitors, and that YKL-40 could be involved in the differentiation of a particular astrocytic lineage.
Keywords: astrocytes; brain barriers; cerebral cortex; development; neural stem cells.
Conflict of interest statement
Figures









References
-
- Abraham H, Pérez-García CG, Meyer G. (2004). P73 and Reelin in Cajal-Retzius Cells of the Developing Human Hippocampal Formation. Cereb Cortex 14:484-495 - PubMed
-
- Alvarez-Buylla A, Seri B, Doetsch F. (2002). Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57:751-758 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

